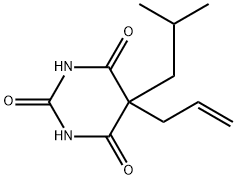
BUTALBITAL
- CAS No.
- 77-26-9
- Chemical Name:
- BUTALBITAL
- Synonyms
- D03182;Technal;Fioricet;Optalidon;Profundal;Sandoptal;Medigesic;Phrenilin;BUTALBITAL;Alisobumal
- CBNumber:
- CB4457316
- Molecular Formula:
- C11H16N2O3
- Molecular Weight:
- 224.26
- MDL Number:
- MFCD00056089
- MOL File:
- 77-26-9.mol
| Melting point | 139-140 °C |
|---|---|
| Boiling point | 365.66°C (rough estimate) |
| Density | 1.1672 (rough estimate) |
| refractive index | 1.5000 (estimate) |
| Flash point | 11 °C |
| storage temp. | 2-8°C |
| solubility | soluble in DMSO, Methanol |
| pka | pKa 12.36±0.05(H2O t=38.0 I=0.1) (Uncertain) |
| form | Solid |
| color | White |
| Water Solubility | 1.702g/L(25 ºC) |
| BRN | 202119 |
| FDA UNII | KHS0AZ4JVK |
| EPA Substance Registry System | 2,4,6(1H,3H,5H)-Pyrimidinetrione, 5-(2-methylpropyl)-5-(2-propen-1-yl)- (77-26-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H317 | |||||||||
| Precautionary statements | P280-P301+P312+P330 | |||||||||
| Hazard Codes | Xn,T,F | |||||||||
| Risk Statements | 22-43-39/23/24/25-23/24/25-11 | |||||||||
| Safety Statements | 36-45-36/37-16 | |||||||||
| RIDADR | UN 1230 3/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | CP8750000 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 2933530000 | |||||||||
| Toxicity | LD50 oral in bird - wild: 75mg/kg | |||||||||
| NFPA 704 |
|
BUTALBITAL Chemical Properties,Uses,Production
Chemical Properties
White, crystalline powder; odorless; slightly bitter taste. Soluble in alcohol, ether, and chloroform; almost insoluble in water.
Originator
Axocet,Savage Labs
Uses
Controlled substance (depressant). Sedative, hypnotic.
Definition
ChEBI: A member of the class of barbiturates that is barbituric acid in which the hydrogens at position 5 are substituted by an allyl group and an isobutyl group. Frequently combined with other medicines, such as aspirin, paracetamol and codeine, it is used for reatment of pain and headache.
Manufacturing Process
1 mole of sodium is dissolved in 10 to 12 times its weight of absolute alcohol under a reflux condenser. To this are added 1 mole of ethyl malonic acid ester,
and then gradually about 1.1 moles of 2-isobutyl bromide. The mixture is
gently refluxed for some hours, or until it no longer shows alkaline reaction to
moist litmus paper. Most of the alcohol is removed by vacuum distillation,
leaving an oily residue. Water is added to this residue to dissolve the sodium
bromide; and the oily layer, which is ethyl isopropyl-carbinyl malonic acid
ester, is separated and dried. It is purified by fractional distillation in vacuum.
When thus purified, ethyl isopropyl-carbinyl malonic acid ester is a colorless or
pale yellow liquid, having a boiling point of 103°-105°C at about 4 mm
pressure, and a refractive index at 25°C.
3 moles of sodium are dissolved in 10 to 12 times its weight of absolute
alcohol under a reflux condenser. To this are added 1.6 moles of urea and 1
mole of ethyl isopropyl-carbinyl malonic acid ester. The mixture is gently
refluxed for 2-4 h, after which most of the alcohol is removed by vacuum
distillation. The residue is dissolved in water, and a sufficient amount of dilute
acid is added to completely precipitate the isopropyl-carbinyl barbituric acid.
The precipitate is filtered off, dried, and recrystallized from dilute alcohol.
1 mole of isopropyl-carbinyl barbituric acid is dissolved in a suitable vessel in
a 10%-35% aqueous solution of 1 mole of potassium hydroxide. To this are
added somewhat in excess of 1 mole of allyl bromide, and alcohol equal to
about 10% of the total volume of the solution. The vessel is agitated for 50-
75 h. At the end of this time, the solution, which may still exhibit two layers,
is concentrated to about one-half its volume, to remove the excess allyl
bromide and the alcohol. On cooling, an oily layer, which is isopropyl-carbinyl
allyl barbituric acid, separates out as a sticky viscous mass. It is dried,
washed with petroleum ether, and dissolved in the minimum amount of
benzene. Any unreacted isopropyl-carbinyl barbituric acid, which does not
dissolve, is filtered off. The addition of petroleum ether to the clear filtrate
causes the isopropyl-carbinyl allyl barbituric acid to precipitate as an oily
mass. This is separated, washed with petroleum ether, and dried in vacuum.
brand name
Sandoptal (Novartis).
Therapeutic Function
Hypnotic, Sedative
Purification Methods
It can be recrystallised from H2O or dilute EtOH, and sublimes at 100-120o/8-12mm. It is soluble in *C6H6, cyclohexane, tetralin and pet ether at 20o. [Butler et al. J Am Chem Soc 77 1486 1955, Beilstein 24 III/IV 2006.]