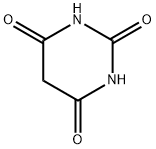
Barbituric acid
- CAS No.
- 67-52-7
- Chemical Name:
- Barbituric acid
- Synonyms
- pyrimidine-2,4,6(1H,3H,5H)-trione;2,4,6(1H,3H,5H)-PYRIMIDINETRIONE;barbituric;MALONYLUREA;PyriMidine-2,4,6-triol;Fluorouracil EP Impurity A;BARBITURIC ACID, FOR THE DETERMINATION O F CYANIDE;Barbitursure;ARBIBTONE ACID;Babituric acid
- CBNumber:
- CB0258599
- Molecular Formula:
- C4H4N2O3
- Molecular Weight:
- 128.09
- MDL Number:
- MFCD00006666
- MOL File:
- 67-52-7.mol
- MSDS File:
- SDS
| Melting point | 248-252 °C (dec.) (lit.) |
|---|---|
| Boiling point | 260℃ (decomposition) |
| Density | 1.6006 (rough estimate) |
| refractive index | 1.4610 (estimate) |
| Flash point | 150 °C |
| storage temp. | Store below +30°C. |
| solubility | 11.45g/l |
| form | Powder/Solid |
| pka | 4.01(at 25℃) |
| color | Light Cream |
| Odor | Odorless |
| PH | 2-3 (50g/l, H2O, 60℃) |
| Water Solubility | 142 g/L (20 ºC) |
| Merck | 14,963 |
| BRN | 120502 |
| InChIKey | HNYOPLTXPVRDBG-UHFFFAOYSA-N |
| CAS DataBase Reference | 67-52-7(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | WQ92Y2793G |
| NIST Chemistry Reference | Barbituric acid(67-52-7) |
| EPA Substance Registry System | Barbituric acid (67-52-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P280a-P304+P340-P305+P351+P338-P405-P501a | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 24/25 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | CP8000000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29335200 | |||||||||
| Toxicity | LD50 orally in Rabbit: > 5000 mg/kg | |||||||||
| NFPA 704 |
|
Barbituric acid price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHR2865 | Fluorouracil Related Compound A certified reference material, pharmaceutical secondary standard | 67-52-7 | 50MG | $485 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.00133 | Barbituric acid for synthesis | 67-52-7 | 100g | $71 | 2024-03-01 | Buy |
| Sigma-Aldrich | 185698 | Barbituric acid 99% | 67-52-7 | 25g | $45.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.00133 | Barbituric acid for synthesis | 67-52-7 | 250g | $148 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1279019 | Fluorouracil Related Compound A United States Pharmacopeia (USP) Reference Standard | 67-52-7 | 25mg | $1210 | 2024-03-01 | Buy |
Barbituric acid Chemical Properties,Uses,Production
Description
Barbiturates are derivatives of barbituric acid, barbituric acid is formed by the condensation of malonic acid and urea, itself has no anesthetic effect, but if its C2 and C5 atoms are substituted by different genes, it can generate many species of barbiturate agents ,for example, oxygen of C2 is replaced by sulfur, which generates sulfur barbiturates, such as thiopental.
Barbiturates'mechanism is basically the same, they act on different levels of the central nervous system, and have a non-specific inhibition. Its sedative and hypnotic effects may be related to selective inhibition of thalamic reticular upstream activating system , thereby blocking the excite transduction to cerebral cortex. Its Anticonvulsant effect is performed through inhibiting synaptic transmission in the central nervous system ,to improve the electrical stimulation threshold in motor cortex.
barbiturates having a therapeutic effect play a inhibiting role in the central nervous system , such as phenobarbital (phenobarbitone), amobarbital (amylobarbitone), thiopental , methohexital (methohexi-tone ). Inhibitory barbiturates have sedative, hypnotic, anticonvulsant and anesthetic effects, but its sedative-hypnotic agent has been eliminated, because in the process it is easy to produce severe tolerance, drug dependence and drug liver enzyme induction.
Because of some differences in their chemical structure, the body eliminate and fat-soluble manner of every drug are different , thus the speed of appearing effect and time of continuing also vary . Long-acting barbiturates such as phenobarbital (phenobarbi-tone) are still used in the treatment of epilepsy anticonvulsant. Ultrashort acting barbiturates (thiopental and methohexital) are often applied as an intravenous anesthetic.
Barbiturate intravenous anesthetics used clinically are now about ten kinds, but three to five species are commonly used . According to the view of Anesthesiology, barbiturates can be divided into two categories, namely hypnotic barbiturates and barbiturate anesthesia. The former are markedly slower drugs such as phenobarbital,having a sedative effect, before anesthesia,its administration can make the patient quiet. After intravenous injection of the latter, consciousness soon disappear,it is mainly used for general anesthesia, in which the most commonly used drug is thiopental, so this drug is representative.
Phenobarbital is a barbituric acid derivative, having weak acid,it is the central inhibitor, mainly inhibiting brain ascending reticular activating system. The shallow to deep degree of inhibition of the drug are due to the amount of small to large ,it has different levels of sedative, hypnotic and anticonvulsant, anesthetic effect. In addition, the drug also has antiepileptic effect.
The above information is edited by the chemicalbook of Tian Ye.
Chemical Properties
cream coloured fine crystalline powder. Odorless. Soluble in water and ether, insoluble in water and alcohol.
Uses
Barbituric acid is widely used in the manufacturing of plastics, textiles, polymers and pharmaceuticals. It is an active ingredient in the production of Vitamin B2. It is a strong acid in an aqueous medium with an active methylene group involved Knoevenegal condensation. It is used as precursor for the preparation of 5-arylidene barbituric acid by reacting with aromatic aldehyde. It is also used in electrochemical oxidation of iodine using cyclic voltammetry and controlled potential coulometry.
Definition
ChEBI: Barbituric acid is a barbiturate, the structure of which is that of perhydropyrimidine substituted at C-2, -4 and -6 by oxo groups. Barbituric acid is the parent compound of barbiturate drugs, although it is not itself pharmacologically active. It has a role as an allergen and a xenobiotic. It is a conjugate acid of a barbiturate, a barbiturate(2-) and a barbiturate(1-).
Preparation
Barbituric acid is derived By the reaction of diethyl malonate and urea. First put Urea in a reaction tank containing methanol ,heat , reflux , dissolve, then add the dried diethyl malonate and sodium methoxide, the reaction is refluxed at 66-68°C for 4-5h, after distillation to recover methanol, cooling to 40-50°C, add dilute hydrochloric acid to adjust to pH 1-2.Cool to room temperature, throw to obtain crude, wash with distilled water once, dry to get crude , and then purify with water and activated carbon, dry to obtain products. Industrial barbituric acid is white or pink crystalline powder, strongly acidic, more than 98% content, melting point ≥245°C. Material consumption fixed: diethyl malonate 1098kg/t, urea 476kg/t, hydrochloric acid (reagent grade III) 681kg/t, sodium methanol (28%) 369kg/t, methanol 1025kg/t.
Application
Barbituric acid is a parent compound of barbiturate drugs. Unsubstituted barbituric acid has no hypnotic properties.
Barbituric acid (BA) may be used in the preparation of the corresponding hemiaminals, via chemoselective reduction in the presence of SmI2/H2O reagent. It may be used in the preparation of BA- modified conjugated carbon nitride nanosheets.
It may be used to synthesize:
5-ylidenebarbituric acid derivatives via Knoevenagel condensation with aromatic and α,β-conjugated aromatic aldehydes
5-diaminomethylenebarbiturates by reacting with substituted carbodiimides
Reactions
Barbituric acid with aromatic aldehydes was used in an experimental study, meant to demonstrate the increased efficiency of Knoevenagel condensation reaction for barbituric acid and various aromatic aldehydes on basic alumina, in the absence of organic solvents under microwave irradiation. It may also be used in electrochemical oxidation of iodine, using cyclic voltammetry and controlled-potential coulometry.
General Description
Barbituric acid is a useful acid for organic and drug syntheses. Its dihydrate form can be synthesized from barbituric acid via crystallization from aqueous solution. Crystal structure of barbituric acid (in tautomeric form) has been investigated by a three dimensional fourier transform method. Its enol crystal form has been reported to be thermodynamically stable.
Purification Methods
Recrystallise it twice from H2O, then dry it for 2 days at 100o. [Beilstein 24 III/IV 1873.]
Barbituric acid Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 | kaia@neputrading.com | China | 1011 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Standardpharm Co. Ltd. | 86-714-3992388 | overseasales1@yongstandards.com | United States | 14336 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
View Lastest Price from Barbituric acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-10-30 | Barbituric acid
67-52-7
|
US $0.00 / kg | 1kg | 99% | 100 tons | Anhui Yiao New Material Technology Co., Ltd | |
 |
2023-01-31 | Barbituric acid
67-52-7
|
US $0.00 / Kg/Drum | 1Kg/Drum | 99% | 5000KG | Hebei Mojin Biotechnology Co., Ltd | |
 |
2022-10-27 | Barbituric acid
67-52-7
|
US $200.00-150.00 / kg | 0.5kg | 99.9% | 10000 | Hebei Nafu Technology Co. , Ltd. |
-

- Barbituric acid
67-52-7
- US $0.00 / kg
- 99%
- Anhui Yiao New Material Technology Co., Ltd
-

- Barbituric acid
67-52-7
- US $0.00 / Kg/Drum
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Barbituric acid
67-52-7
- US $200.00-150.00 / kg
- 99.9%
- Hebei Nafu Technology Co. , Ltd.