Dichloromethane
- CAS No.
- 75-09-2
- Chemical Name:
- Dichloromethane
- Synonyms
- DCM;METHYLENE CHLORIDE;CH2Cl2;Methylene dichloride;Dichlormethan;F30;Methylenchlorid;DichL;Metaclen;Dichlorome
- CBNumber:
- CB7740372
- Molecular Formula:
- CH2Cl2
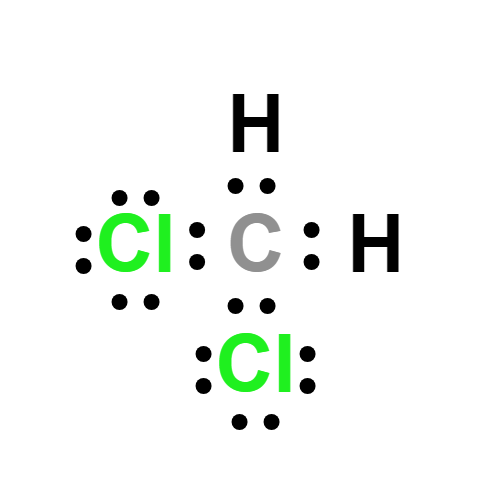
Lewis structure
- Molecular Weight:
- 84.93
- MDL Number:
- MFCD00672695
- MOL File:
- 75-09-2.mol
- MSDS File:
- SDS
| Melting point | -97 °C |
|---|---|
| Boiling point | 39.8-40 °C mm Hg(lit.) |
| Density | 1.325 g/mL at 25 °C(lit.) |
| vapor density | 2.9 (vs air) |
| vapor pressure | 24.45 psi ( 55 °C) |
| refractive index |
n |
| Flash point | 39-40°C |
| storage temp. | room temp |
| solubility | Miscible in ethyl acetate, alcohol, hexanes, methanol, diethyl ether, n-octanol, acetone benzene, carbon tetrachloride, diethyl ether and chloroform. |
| form | Liquid |
| Specific Gravity | 1.329 (20/20℃) |
| color | APHA: ≤10 |
| Odor | Odor threshold 160 to 230 ppm |
| Odor Threshold | 160ppm |
| explosive limit | 13-22%(V) |
| Evaporation Rate | 0.71 |
| Water Solubility | 20 g/L (20 ºC) |
| λmax |
λ: 235 nm Amax: 1.00 λ: 240 nm Amax: 0.20 λ: 250 nm Amax: 0.05 λ: 260 nm Amax: 0.02 λ: 340-400 nm Amax: 0.01 |
| Merck | 14,6063 |
| BRN | 1730800 |
| Henry's Law Constant | 2.49 at 30 °C (headspace-GC, Sanz et al., 1997) |
| Dielectric constant | 9.1(20℃) |
| Exposure limits | TLV-TWA 50 ppm (~175 mg/m3) (ACGIH); carcinogenicity: Suspected Human Carcinogen (ACGIH), Animal Sufficient Evidence, Human Inadequate Evidence (IARC). |
| Stability | Volatile |
| EPA Primary Drinking Water Standard | MCL:0.005,MCLG:zero |
| LogP | 1.250 |
| Substances Added to Food (formerly EAFUS) | METHYLENE CHLORIDE |
| CAS DataBase Reference | 75-09-2(CAS DataBase Reference) |
| EWG's Food Scores | 5-8 |
| FDA UNII | 588X2YUY0A |
| Proposition 65 List | Dichloromethane (Methylene Chloride) |
| NIST Chemistry Reference | Methylene chloride(75-09-2) |
| IARC | 2A (Vol. Sup 7, 71, 110) 2017 |
| EPA Substance Registry System | Methylene chloride (75-09-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H336-H351 | |||||||||
| Precautionary statements | P201-P202-P261-P302+P352-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | Xn,T,F,N,C | |||||||||
| Risk Statements | 40-39/23/24/25-23/24/25-11-67-36/37/38-68/20/21/22-20/21/22-50-37-34 | |||||||||
| Safety Statements | 23-24/25-36/37-45-16-7-26-61-36/37/39 | |||||||||
| RIDADR | UN 1593 6.1/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | PA8050000 | |||||||||
| F | 3-10 | |||||||||
| Autoignition Temperature | 556 °C | |||||||||
| Hazard Note | Harmful | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2903 12 00 | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| Toxicity | LD50 orally in young adult rats: 1.6 ml/kg (Kimura) | |||||||||
| IDLA | 2,300 ppm | |||||||||
| NFPA 704 |
|
Dichloromethane price More Price(157)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | DX0838 | Dichloromethane HPLC, Meets ACS Specifications, Meets Reagent Specifications for testing USP/NF monographs | 75-09-2 | 1L | $105 | 2024-03-01 | Buy |
| Sigma-Aldrich | DX0838 | Dichloromethane HPLC, Meets ACS Specifications, Meets Reagent Specifications for testing USP/NF monographs | 75-09-2 | 4L | $223 | 2024-03-01 | Buy |
| Sigma-Aldrich | DX0835 | Dichloromethane Meets ACS Specifications, Meets Reagent Specifications for testing USP/NF monographs GR ACS | 75-09-2 | 500mL | $106 | 2024-03-01 | Buy |
| Sigma-Aldrich | DX0838 | Dichloromethane HPLC, Meets ACS Specifications, Meets Reagent Specifications for testing USP/NF monographs | 75-09-2 | 18.9L | $2520 | 2024-03-01 | Buy |
| Sigma-Aldrich | DX0835 | Dichloromethane Meets ACS Specifications, Meets Reagent Specifications for testing USP/NF monographs GR ACS | 75-09-2 | 1L | $211 | 2024-03-01 | Buy |
Dichloromethane Chemical Properties,Uses,Production
Overview
Dichloromethane (DCM), also known as methylene chloride, is a transparent, colorless, volatile halogenated aliphatic hydrocarbon compound with an ether-like mildly sweet smell. It is moderately soluble in water as well as in most organic solvents namely; ether, ethanol, ketones, aldehydes, and phenols (1). Notably, DCM vapors are heavier than air and are normally non-explosive, stable, and non-flammable when exposed in the air; however, temperatures above 100oC must be avoided. Although natural sources do not largely contribute to the global release of DCM, the latter may lead to the formation of the former.
Chemical Properties
Methylene chloride reacts strongly with active metals such as potassium, sodium, and lithium, and strong bases, for instance, potassium tert-butoxide. However, the compound is incompatible with strong caustics, strong oxidizers, and metals that are chemically active such as magnesium and aluminum powders.
It is noteworthy that methylene chloride can attack some forms of coatings, plastic, and rubber. In addition, dichloromethane reacts with liquid oxygen, sodium-potassium alloy, and nitrogen tetroxide. When the compound comes into contact with water, it corrodes some stainless steels, nickel, copper as well as iron.
When exposed to heat or water, dichloromethane becomes very sensitive as it is subjected to hydrolysis that is hastened by light. Under normal conditions, solutions of DCM such as acetone or ethanol should be stable for 24 hours.
Methylene chloride does not react with alkali metals, zinc, amines, magnesium, as well as alloys of zinc and aluminum. When mixed with nitric acid or dinitrogen pentoxide, the compound can vigorously explode. Methylene chloride is flammable when mixed with methanol vapor in the air.
Since the compound can explode, it is important to avoid certain conditions such as sparks, hot surfaces, open flames, heat, static discharge, and other ignition sources.
Uses
House Hold Uses
The compound is used in bathtub refurbishing. Dichloromethane is highly used industrially in the production of pharmaceuticals, strippers, and process solvents.
Industrial and Manufacturing Uses
DCM is a solvent that is found in varnish and paint strippers, which are often used to remove varnish or paint coatings from various surfaces. As a solvent in the pharmaceutical industry, DCM is used for the preparation of cephalosporin and ampicillin.
Food and Beverage Manufacturing
It is also used in manufacturing beverage and food manufacturing as an extraction solvent. For instance, DCM can be used to decaffeinate unroasted coffee beans as well as tea leaves. The compound is also used in creating hops extract for beer, beverages and other flavoring for foods, as well as in processing spices.
Transportation Industry
DCM is normally used in the degreasing of metal parts and surfaces, such as railroad equipment and tracks as well as airplane components. It can also be used in degreasing and lubricating products utilized in automotive products, for instance, removal of the gasket and for preparing metal parts for a new gasket.
Experts in automotive commonly use vapor dichloromethane degreasing process to for the removal of grease and oils from car parts of car transistor, spacecraft assemblies, aircraft components, and diesel motors. Today, specialists are able to safely and quickly clean transportation systems using degreasing techniques that depend on methylene chloride.
Medical Industry
Dichloromethane is used in laboratories in the extraction of chemicals from foods or plants for medicines such as antibiotics, steroids, and vitamins. In addition, medical equipment can be efficiently and quickly cleaned using dichloromethane cleaners while avoiding damage to heat-sensitive parts and corrosion problems.
Photographic Films
Methylene chloride is used as a solvent in the production of cellulose triacetate (CTA), which is applied in the creation of safety films in photography. When dissolved in DCM, CTA begins to evaporate as the fibre of acetate remains behind.
Electronic Industry
Methylene chloride is used in the production of printed circuit boards in the electronic industry. DCM is utilized to degrease the foil surface of the substrate before the photoresist layer is added to the board.
Detriment
Dichloromethane enters the human body mainly through inhalation and can cause anesthetic effects such as damages to the respiratory system and the central nervous system. When being used as a paint remover, DCM has been found to be present in high concentrations in indoor environments. The compound can be exposed to the general population through drinking water, air, and food contact, albeit in much smaller levels. Moreover, it is impossible for the compound to accumulate in the atmosphere due to its photolysis rate. Workers who are engaged in the manufacture of DCM, polycarbonate resin and paint remover formulation are at high risk of exposure.
Content analysis
Dichloromethane can be separated by dibutyl phthalate (DBP), then detected by GC with TCD, and quantified by comparison with standard dichloromethane.
Reagents: carrier gas, helium (> 99.5%); carrier for white diatomaceous earth 6201 (40-60 mesh) or equivalent; stationary phase, DBP (in ether); standard dichloromethane, chromatographically pure dichloromethane;
Instruments: a gas chromatograph with TCD, a column, a 3 m x 3 to 4 mm (inner diameter) stainless steel column.
Conditions: fixed phase, 20%:DBP/6021 gasification temperature, 100℃, detection temperature 100℃; carrier gas flow rate, 70ml/min, column temperature of 70 ℃; TCD bridge current, 200mA ~; injection volume, <20μL; temperature. retention time (R) of other chlorinated solvents related to dichloromethane: methyl chloride 0.15; monochloroethane 0.34; 1,1-dichloroethylene 0.59; monochloropropylene, 0.85; carbon tetrachloride 1.86; chloroform 2.47.
Toxicity
ADI gives no specific stipulation (the residual amount of dichloromethane in the products should be minimized as long as the production demand is meet;FAO/WHO。1998)。
Hazards & Safety Information
toxicity grade:WHO Class II
acute toxicity:acute peroral LD50 in rats 1600 mg/kg; mouse intraperitoneal LD50: 437 mg/kg
Physiological stimulation:skin-rabbit 810 mg/24hours Severe; eyes-rabbit 500 mg/24hours Mild
Explosive hazard characteristics:Explosive when mixed with air or oxygen
Combustible hazard characteristics:It releases phosgene when heated. Its vapor is non-flammable
transportation and storing characteristics:In ventilated dry storeroom at low temperature, kept apart from oxidizing agent and nitric acid
extinguishant: Foam extinguisher, carbon dioxide, sprayed water, yellow sand.
professional standard: TWA 350 mg/m3;STEL 879 mg/m3.
Description
Dichloromethane is a colorless liquid with an ethereal, but penetrating odor. Its miscibility in alcohol and ether and slight solubility in water has made it an ideal solvent and otherwise extremely versatile chemical. It has been used industrially (solvent and paint remover), as a drug (inhalation anesthetic) and as an agricultural chemical (growth regulator and fertilizer). It is narcotic in high concentrations and carcinogenic. Inhalation exposure to this substance irritates the nose and throat and affects the central nervous system.
Chemical Properties
Dichloromethane is a colorless liquid with a mild, sweet odor. It does not occur naturally in the environment. It is made from methane gas or wood alcohol. Industrial uses of dichloromethane are extensive, as a solvent in paint strippers, as a propellant in aerosols, and as a process solvent in the manufacturing of drugs. dichloromethane is also used as a metal cleaning and fi nishing solvent, and it is approved as an extraction solvent for spices and hops. Exposure to dichloromethane occurs in workplaces by breathing fumes from paint strippers that contain it (check the label), breathing fumes from aerosol cans that use it (check the label), and breathing contaminated air near waste sites.
Physical properties
Clear, colorless liquid with a sweet, penetrating, ethereal odor. Leonardos et al. (1969) determined an odor threshold concentration of 214.0 ppmv. The average least detectable odor threshold concentrations of technical grade methylene chloride in water at 60 °C and in air at 40 °C were 5.6 and 24 mg/L, respectively (Alexander et al., 1982).
Uses
Methylene chloride is primarily used as a paint remover solvent. It is also used as an aerosol propellant, processing solvent in the production of steroids, antibiotics, vitamins, and tablet coatings. Additionally, it serves as a degreasing agent, particularly in electronics manufacturing. Methylene chloride is utilized as a urethane foamblowing agent as well. It finds application in metal cleaning, production of polycarbonate resins and triacetate fibers, film processing, ink formulations, and as an extraction solvent for spice oleoresins, caffeine, and hops. Due to its high volatility, it is commonly employed as a solvent in various extraction processes. Methylene chloride possesses strong solvent power for cellulose esters, fats, oils, resins, and rubber. It is more soluble in water compared to other chlorinated solvents. In the past, it was approved for use as an insecticide for commodity fumigation of strawberries, citrus fruits, and grains.
Uses
Dichloromethane, also called methylene chloride, is widely used as a solvent, as a degreasing and cleaning reagent, in paint removers, and in extractions oforganic compounds from water for analyses.
Definition
ChEBI: Dichloromethane is a member of the class of chloromethanes that is methane in which two of the hydrogens have been replaced by chlorine. A dense, non-flammible colourless liquid at room temperature (b.p. 40℃, d = 1.33) which is immiscible with water, it is widely used as a solvent, a paint stripper, and for the removal of caffeine from coffee and tea. It has a role as a polar aprotic solvent, a carcinogenic agent and a refrigerant. It is a member of chloromethanes and a volatile organic compound.
Production Methods
Dichloromethane was first prepared by Regnault in 1840 by the chlorination of methyl chloride in sunlight. It became an industrial chemical of importance during the Second World War. Two commercial processes are currently used for the production of dichloromethane—hydrochlorination of methanol and direct chlorination of methane (Rossberg et al., 1986; Holbrook, 1993). The predominant method of manufacturing dichloromethane uses as a first step the reaction of hydrogen chloride and methanol to give methyl chloride. Excess methyl chloride is then mixed with chlorine and reacts to give dichloromethane, with chloroform and carbon tetrachloride as co-products. This reaction is usually carried out in the gas phase thermally but can also be performed catalytically or photolytically. At low temperature and high pressure, the liquid-phase process is capable of giving high selectivity for dichloromethane (Rossberg et al., 1986; Holbrook, 1993).
Reactions
Methylene chloride reacts violently in the presence of alkali or alkaline earth metals and will hydrolyze to formaldehyde in the presence of an aqueous base. Alkylation reactions occur at both functions, thus di-substitutions result.
General Description
Dichloromethane has been tested as a solvent medium for the dipyridine-chromium(VI) oxide. Solubility was reported to be 12.5g/100ml. Role of quantity of TiO2 loading on activated carbon support employed in the photodecomposition of dichloromethane has been investigated.
Air & Water Reactions
Methylene chloride is a colourless liquid with a mild, sweet odour. Somewhat water soluble. Subject to slow hydrolysis which is accelerated by light.
Reactivity Profile
Dichloromethane reacts vigorously with active metals such as lithium, sodium and potassium, and with strong bases such as potassium tert-butoxide. Dichloromethane is incompatible with strong oxidizers, strong caustics and chemically active metals such as aluminum or magnesium powders. The liquid will attack some forms of plastic, rubber and coatings. Dichloromethane reacts with sodium-potassium alloy, (potassium hydrogen + N-methyl-N-nitrosurea), nitrogen tetraoxide and liquid oxygen. Dichloromethane also reacts with titanium. On contact with water Dichloromethane corrodes iron, some stainless steels, copper and nickel. Dichloromethane is incompatible with alkali metals. Dichloromethane is incompatible with amines, zinc and alloys of aluminum, magnesium and zinc. Dichloromethane is liable to explode when mixed with dinitrogen pentaoxide or nitric acid. Mixtures of Dichloromethane in air with methanol vapor are flammable.
Hazard
Toxic. A narcotic. Central nervous systemimpairment and carboxyhemoglobinemia. Possiblecarcinogen.
Health Hazard
Dichloromethane is classified as only slightly toxic by the oral and inhalation routes. Exposure to high concentrations of dichloromethane vapor (>500 ppm for 8 h) can lead to lightheadedness, fatigue, weakness, and nausea. Contact of the compound with the eyes causes painful irritation and can lead to conjunctivitis and corneal injury if not promptly removed by washing. Dichloromethane is a mild skin irritant, and upon prolonged contact (e.g., under the cover of clothing or shoes) can cause burns after 30 to 60 min exposure. Dichloromethane is not teratogenic at levels up to 4500 ppm or embryotoxic in rats and mice at levels up to 1250 ppm.
Fire Hazard
Special Hazards of Combustion Products: Dissociation products generated in a fire may be irritating or toxic.
Flammability and Explosibility
Noncombustible. Dichloromethane vapor concentrated in a confined or poorly ventilated area can be ignited with a high-energy spark, flame, or high-intensity heat source.
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Confirmed carcinogen with experimental carcinogenic and tumorigenic data. Poison by intravenous route. Moderately toxic by ingestion, subcutaneous, and intraperitoneal routes. Mildly toxic by inhalation. Human systemic effects by ingestion and inhalation: paresthesia, somnolence, altered sleep time, convulsions, euphoria, and change in cardlac rate. An experimental teratogen. Experimental reproductive effects. An eye and severe skin irritant. Human mutation data reported. It is flammable in the range of 12-19% in air but ignition is difficult. It will not form explosive mixtures with air at ordinary temperatures. Mixtures in air with methanol vapor are flammable. It will form explosive mixtures with an atmosphere having a high oxygen content, in liquid O2, N2O4, K, Na, NaK. Explosive in the form of vapor when exposed to heat or flame. Reacts violently with Li, NaK, potassiumtert- butoxide, (KOH + N-methyl-Nnitrosourea). It can be decomposed by contact with hot surfaces and open flame, and then yield toxic fumes that are irritating and give warning of their presence. When heated to decomposition it emits highly toxic fumes of phosgene and Cl-.
Potential Exposure
Methylene chloride is used mainly as a low-temperature extractant of substances which are adversely affected by high temperature. It can be used as a solvent for oil, fats, waxes, bitumen, cellulose acetate; and esters. It is also used as a paint remover; as a degreaser; and in aerosol propellants
First aid
If this chemical gets into the eyes, remove anycontact Tenses at once and irrigate immediately for at least5 min, occasionally lifing upper and lower lids. Seek medi-cal attention immediately. If this chemical contacts the skin,remove contaminated clothing and wash immediately withsoap and water. Seek medical attention immediately. If thisChemical has been inhaled, remove from exposure, begin res-cue breathing (using universal precautions, including resusci-ation mask) if breathing has stopped and CPR if heart actionhas stopped. Transfer promptly to a medical facility. Whenthis chemical has been swallowed, get medical attention.Give large quantities of water and induce vomiting. Do notmake an unconscious person vomit. Medical observation isrecommended for 24- 48 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmonaryedema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.
Carcinogenicity
Dichloromethane is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Environmental Fate
Biological. Complete microbial degradation to carbon dioxide was reported under anaerobic
conditions by mixed or pure cultures. Under enzymatic conditions formaldehyde was the only
product reported (Vogel et al., 1987). In a static-culture-flask screening test, methylene chloride (5
and 10 mg/L) was statically incubated in the dark at 25 °C with yeast extract and settled domestic
wastewater inoculum. After 7 d, 100% biodegradation with rapid adaptation was observed (Tabak
et al., 1981).
Under aerobic conditions with sewage seed or activated sludge, complete biodegradation was
observed between 6 h to 1 wk (Rittman and McCarty, 1980).
Soil. Methylene chloride undergoes biodegradation in soil under aerobic and anaerobic
conditions. Under aerobic conditions, the following half-lives were reported: 54.8 d in sand (500
ppb); 1.3, 9.4, and 191.4 d at concentrations of 160, 500, and 5,000 ppb, respectively, in sandy
loam soil; 12.7 d (500 ppb) in sandy clay loam soil; 7.2 d (500 ppb) following a 50-d lag time.
Under anaerobic conditions, the half-life of methylene chloride in clay following a 70-d lag time is
21.5 d (Davis and Madsen, 1991). The estimated volatilization half-life of methylene chloride in
soil is 100 d (Jury et al., 1990).
Photolytic. Reported photooxidation products via OH radicals include carbon dioxide, carbon
monoxide, formyl chloride, and phosgene (Spence et al., 1976). In the presence of water, phosgene
hydrolyzes to HCl and carbon dioxide, whereas formyl chloride hydrolyzes to hydrogen chloride
and carbon monoxide (Morrison and Boyd, 1971).
Chemical/Physical. Under laboratory conditions, methylene chloride hydrolyzed with
subsequent oxidation and reduction to produce methyl chloride, methanol, formic acid, and
formaldehyde (Smith and Dragun, 1984). The experimental half-life for hydrolysis in water at 25
°C is approximately 18 months (Dilling et al., 1975).
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Before entering confined space where this chemicalmay be present, check to make sure that an explosive concentration does not exist. Methylene chloride must be storedto avoid contact with strong oxidizers (such as perchlorates,peroxides, chlorates, nitrates, or permanganates), strongcaustics, and chemically active metals (such as aluminum,magnesium powder, sodium, potassium, or lithium) becauseviolent reactions occur. Store in tightly closed containers ina cool, well-ventilated area away from heat and moisture. Aregulated, marked area should be established where thischemical is handled, used, or stored in compliance withOSHA Standard 1910.1045.
Shipping
UN1593Dichloromethane, Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Purification Methods
Shake it with portions of conc H2SO4 until the acid layer remains colourless, then wash with water, aqueous 5% Na2CO3, NaHCO3 or NaOH, then water again. Pre-dry with CaCl2, and distil it from CaSO4, CaH2 or P2O5. Store it away from bright light in a brown bottle with Linde type 4A molecular sieves, in an atmosphere of dry N2. Other purification steps include washing with aqueous Na2S2O3, passage through a column of silica gel, and removal of carbonyl-containing impurities as described under Chloroform. It has also been purified by treatment with basic alumina, distillation, and stored over molecular sieves under nitrogen [Puchot et al. J Am Chem Soc 108 2353 1986]. Dichloromethane from Japanese sources contained MeOH as stabiliser which is not removed by distillation. It can, however, be removed by standing over activated 3A Molecular Sieves (note that 4A Sieves cause the development of pressure in bottles), passed through activated Al2O3 and distilled [Gao et al. J Am Chem Soc 109 5771 1987]. It has been fractionated through a platinum spinning band column, degassed, and distilled onto degassed molecular sieves Linde 4A (heated under high vacuum at over 450o until the pressure readings reached the low values of 10-6 mm, ~1-2hours ). Stabilise it with 0.02% of 2,6-di-tert-butyl-p-cresol [Mohammad & Kosower J Am Chem Soc 93 2713 1971]. [Beilstein 1 IV 35.] Rapid purification: Reflux over CaH2 (5% w/v) and distil it. Store it over 4A molecular sieves.
Toxicity evaluation
Dichloromethane is usually released to the atmosphere. It can
react withhydroxyl radicals with a half-life of about a fewmonths.
Dichloromethane released to water can be evaporated to atmosphere
with a half-life of 35.6 h at moderate mixing conditions.
Some of dichloromethane in water can be biodegraded
completely within several hours and a few days. Small part of
dichloromethane released to water can be degraded by hydrolysis.
However, hydrolysis is not an important process under
natural condition and may take 18 months or more to degrade
completely. Dichloromethane released to soil will go to the soil
surface and then the atmosphere. Some part of dichloromethane
in soil will leak to the groundwater and water cycle.
DCM’s production and use as solvent, chemical intermediate,
grain fumigant, paint stripper and remover,metal degreaser, and
refrigerant may result in its release to the environment through
various waste streams. Vapor-phase DCM is expected to be
degraded in the atmosphere by reaction with photochemically
produced hydroxyl radicals; the half-life for this reaction in air is
estimated to be approximately 119 days (in the absence of direct
photolysis). If released to soil,DCMis expected to have very high mobility based on an estimated Koc of 24. Volatilization
from moist soil surfaces is expected to be an important fate
process based on an estimated Henry’s law constant of
3.25×10-3 atm-m3 mol-1. DCM may volatilize from dry soil
surfaces based on its vapor pressure. Biodegradation in soil may
occur. DCM, when released into water, is not expected to adsorb
to suspended solids and sediment in water based on the estimated
Koc. Biodegradation is possible in natural waters but will
probably be very slow compared with evaporation.
Incompatibilities
Incompatible with strong oxidizers, caustics; chemically active metals, such as aluminum, magnesium powders; potassium, lithium, and sodium; concentrated nitric acid causing fire and explosion hazard. Contact with hot surfaces or flames causes decomposition producing fumes of hydrogen chloride and phosgene gas. Attacks some forms of plastics, rubber and coatings. Attacks metals in the presence of moisture.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration, preferably after mixing with another combustible fuel; care must be exercised to assure complete combustion to prevent the formation of phosgene; an acid scrubber is necessary to remove the halo acids produced.
Regulations
Several jurisdictions have acted to reduce the use and release of various volatile organic compounds, including dichloromethane. The California Air Resources Board was one of the first jurisdictions to regulate dichloromethane; in 1995, it limited the levels of total volatile organic compounds (VOCs) contained in aerosol coating products. Subsequent regulations prevented manufacture, sale, supply, or application of any aerosol coating product containing dichloromethane (Air Resources Board, 2001). California has also prohibited the manufacture, sale, or use of automotive cleaning and degreasing products containing dichloromethane.
In Japan, the environmental quality standards for dichloromethane state that outdoor air levels shall not exceed 0.15 mg/m3 (Ministry of the Environment Government of Japan, 2014).
A guideline value of 3 mg/m3 for 24-hour exposure is recommended by WHO. In addition, the weekly average concentration should not exceed one seventh (0.45 mg/m3) of this 24-hour guideline (WHO, 2000).
In the European Union, the VOC Solvent Emissions Directive (Directive 1999/13/EC) was implemented for new and existing installations on 31 October 2007 (European Commission,1999). The Directive aims to reduce industrial emissions of VOCs from solvent-using activities, such as printing, surface cleaning, vehicle coating, dry cleaning, and manufacture of footwear and pharmaceutical products. Installations conducting such activities are required to comply either with emission limit values or with a reduction scheme. Reduction schemes allow the operator to reduce emissions by alternative means, such as by substituting products with a lower solvent content or changing to solvent-free production processes. The Solvents Directive was implemented in 2010 into the Industrial Emission Directive 2010/75/EU (IED).
Dichloromethane Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shandong Yanshuo Chemical Co., Ltd. | +86-18678179670 +86-18615116763 | sales@yanshuochem.com | China | 101 | 58 |
| Lihe Pharm Technology(Wuhan)Co.,Ltd | +8618071517867 | info@lihepharma.com | China | 194 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 | peter68@ptchemgroup.com | China | 35453 | 58 |
| Hebei Dangtong Import and export Co LTD | +8615632927689 | admin@hbdangtong.com | China | 991 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +8615271838296 | kyra@quanjinci.com | China | 1532 | 58 |
| Wuhan Ruichi Technology Co., Ltd | +8613545065237 | admin@whrchem.com | China | 164 | 58 |
| Qingdao Minzhi Yijie new material Co., LTD | +86-13589435123 +86-13589435123 | qdmzyj@126.com | China | 240 | 58 |
| Nanjing Deda New Material Technology Co., Ltd | +8613223293093 | bella@njdeda.com | China | 81 | 58 |
| Firsky International Trade (Wuhan) Co., Ltd | +8615387054039 | admin@firsky-cn.com | China | 436 | 58 |
| Nanjing Deda New Material Technology Ltd. | +8613223281135 | niki@njdeda.com | China | 76 | 58 |
Related articles
- Is CH2Cl2 Polar or Nonpolar?
- An electronegativity difference of 0.61 units exists between a carbon and a chlorine atom in the C-Cl bond,makes dichlorometha....
- Dec 20,2023
- Toxicity of Dichloromethane
- Dichloromethane (DCM; mol. wt. 93.328) was first prepared in 1840 by mixing chloromethane and chlorine and exposed to sunshine....
- Jan 18,2022
- Toxicity hazards of Dichloromethane
- Dichloromethane can be used as a solvent, dental local anesthetic, refrigerant and fire extinguishing agent. In addition to be....
- Nov 18,2021
View Lastest Price from Dichloromethane manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-27 | Dichloromethane
75-09-2
|
US $1.00 / kg | 1kg | 99% | 20 tons | Dorne Chemical Technology co. LTD | |
 |
2024-04-26 | Dichloromethane
75-09-2
|
US $10.00-5.00 / kg | 1kg | 99.5 | 10 ton per month | Nanjing Deda New Material Technology Co., Ltd | |
 |
2024-04-26 | Dichloromethane
75-09-2
|
US $10.00-5.00 / kg | 1kg | 99.5 | 10 ton per month | Nanjing Deda New Material Technology Co., Ltd |
-

- Dichloromethane
75-09-2
- US $1.00 / kg
- 99%
- Dorne Chemical Technology co. LTD
-

- Dichloromethane
75-09-2
- US $10.00-5.00 / kg
- 99.5
- Nanjing Deda New Material Technology Co., Ltd
-

- Dichloromethane
75-09-2
- US $10.00-5.00 / kg
- 99.5
- Nanjing Deda New Material Technology Co., Ltd