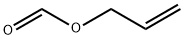ALLYL FORMATE
- CAS No.
- 1838-59-1
- Chemical Name:
- ALLYL FORMATE
- Synonyms
- ALLYL FORMATE;2-propenylformate;Formic acid allyl;prop-2-enyl formate;formicacid,allylester;Allyl alcohol formate;prop-2-enyl methanoate;1838-59-1 ALLYL FORMATE;Formic acid, allyl ester;formicacid,2-propenylester
- CBNumber:
- CB8285997
- Molecular Formula:
- C4H6O2
- Molecular Weight:
- 86.09
- MDL Number:
- MFCD00046144
- MOL File:
- 1838-59-1.mol
- MSDS File:
- SDS
| Melting point | -84.81°C (estimate) |
|---|---|
| Boiling point | 85℃ |
| Density | 0.922 |
| refractive index | 1.4009 (estimate) |
| Flash point | 7℃ |
| Odor | ethereal fruity |
| LogP | 0.601 (est) |
| FDA UNII | A9WY6Q0U9C |
| EPA Substance Registry System | Formic acid, 2-propenyl ester (1838-59-1) |
SAFETY
Risk and Safety Statements
| RIDADR | 2336 |
|---|---|
| HazardClass | 3.1 |
| PackingGroup | I |
| Toxicity | mouse,LC50,inhalation,610mg/m3 (610mg/m3),BEHAVIORAL: GENERAL ANESTHETICBEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY),Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 28(6), Pg. 55, 1984. |
ALLYL FORMATE Chemical Properties,Uses,Production
Description
Allyl formate (CAS # 1838-59-1) is a clear, colorless liquid. It is slightly soluble in water. It is reported to cause liver and kidney injury in animals. The most common effect in humans following occupational exposure to allyl formate exposure is upper respiratory tract irritation.
Uses
Allyl formate is used as a solvent in spray lacquers, enamels, varnishes, and latex paints and as an ingredient in paint thinners and strippers, varnish removers, and herbicides. It is also used in liquid soaps, cosmetics, industrial and household cleaners, and dry-cleaning compounds.
General Description
A colorless liquid. Soluble in water. Flash point between 5 and 67°F. Highly toxic by ingestion, inhalation and skin contact.
Air & Water Reactions
Highly flammable. Soluble in water.
Reactivity Profile
ALLYL FORMATE is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.
Health Hazard
TOXIC; may be fatal if inhaled, ingested or absorbed through skin. Inhalation or contact with some of these materials will irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion and poison hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Poison by ingestion. Moderately toxic by inhalation. Very flammable and reactive. Dangerous fire hazard. See also ALLYL COMPOUNDS and ESTERS. When heated to decomposition it yields irritating smoke and fumes
Environmental Fate
Allyl formate is cleaved by nonspecific esterases to allyl alcohol, which is then oxidized by alcohol dehydrogenases to the reactive acrolein, which is responsible for the hepatotoxic action. The toxicity of allyl alcohol via its metabolite acrolein is dependent on the concentration of GSH. After depletion of GSH, the reactive metabolite of allyl alcohol can bind to essential sulfhydryl groups in the cellular macromolecules, leading to structural and functional modifications, which can be responsible for hepatic injury. Appearance of lipid peroxidation signals events that follow toxication mechanisms initiated by acrolein, and subsequent and continued lipid peroxidation could be merely the consequence of the cell death.
ALLYL FORMATE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 15093356674; | laboratory@coreychem.com | China | 30255 | 58 |
| CD Chemical Group Limited | +8615986615575 | info@codchem.com | China | 20356 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9116 | 58 |
| Shijiazhuang Lida Chemical Co., Ltd. | +86 (311) 8573-7996 | service@lid-ff.cn | China | 294 | 65 |
| Chengdu Ai Keda Chemical Technology Co., Ltd. | 4008-755-333 18080918076 | 800078821@qq.com | China | 9725 | 55 |
| Chengdu HuaXia Chemical Reagent Co. Ltd | 400-1166-196 13458535857 | cdhxsj@163.com | China | 13358 | 58 |
Related articles
- The use of Allyl formate
- Allyl formate is a clear, colorless liquid. It is slightly soluble in water. It is reported to cause liver and kidney injury i....
- Nov 1,2021