- Rapamycin
-

- $999.00/ kg
-
2024-09-20
- CAS:53123-88-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000
- Sirolimus(Rapamycin)
-

- $0.00 / 10g/Bag
-
2024-09-18
- CAS:53123-88-9
- Min. Order: 10g
- Purity: 98%min
- Supply Ability: 20kg/month
- Sirolimus;Rapamycin
-

- $60.00 / 10g
-
2024-09-18
- CAS:53123-88-9
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 25kg
Related articles - Adverse effects of Rapamycin
- Rapamycin, also known as Sirolimus and sold under the brand name Rapamune among others, is a macrolide compound that is used t....
- Apr 14,2022
- What is sirolimus used for?
- Sirolimus is used together with other medicines to prevent the body from rejecting a transplanted kidney. It belongs to a grou....
- Sep 9,2021
- What is Rapamycin?
- Sirolimus, also known as rapamycin, is a macrolide derived from Streptomyces hygroscopicus. Sirolimus and its analog molecules....
- Feb 10,2020
Question and answer - Q:Do any foods contain rapamycin?
- A:Rapamycin is a natively appearing compound. It was originally found in the soil of Easter Island. However, there is no nativel....
- Apr 10,2024
|
| Product Name: | Rapamycin | | Synonyms: | AY 22989;23,27-EPOXY-3H-PYRIDO(2,1-C)(1,4)OXAAZACYCLOHENTRIACONTINE;NSC-226080;RAPAMYCIN;RAPAMYCIN, STREPTOMYCES HYGROSCOPICUS;RPM;antibioticay22989;(3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-Hexadecahydro-9,27-dihydroxy-3-[(1R)-2-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine-1,5,11,28,29(4H,6H,31H)-pentone | | CAS: | 53123-88-9 | | MF: | C51H79NO13 | | MW: | 914.17 | | EINECS: | 610-965-5 | | Product Categories: | VIVACTIL;antibiotic;Inhibitor;Chiral Reagents;Heterocycles;API;Signalling;Immunosuppressant;All Inhibitors;Inhibitors;Intermediates & Fine Chemicals;Pharmaceuticals;Cytokine signaling;Active Pharmaceutical Ingredients;11;53123-88-9 | | Mol File: | 53123-88-9.mol | 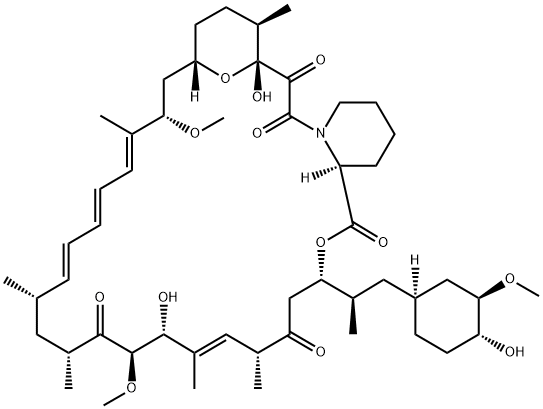 |
| | Rapamycin Chemical Properties |
| Melting point | 183-185°C | | alpha | D25 -58.2° (methanol) | | Boiling point | 799.83°C (rough estimate) | | density | 1.0352 (rough estimate) | | vapor pressure | 0.56 hPa ( 20 °C) | | refractive index | 1.5280 (estimate) | | Fp | 87 °C | | storage temp. | -20°C | | solubility | ethanol: soluble2MM | | pka | 10.40±0.70(Predicted) | | form | solution | | color | colorless to yellow | | Water Solubility | Soluble in DMSO at 50mg/ml or methanol at 25mg/mlSoluble in alcohol, dimethyl sulfoxide and dimethyl formamide. Insoluble in water. | | Sensitive | Moisture Sensitive/Light Sensitive/Hygroscopic | | Merck | 14,8114 | | Stability: | Stable for 2 years? from date of purchase as supplied.? Solutions in DMSO or ethanol may be stored at -20°C for up to 2 months. | | CAS DataBase Reference | 53123-88-9(CAS DataBase Reference) |
| | Rapamycin Usage And Synthesis |
| description |
Rapamycin (also known as Sirolimus; CAS: 53123-88-9) is macrocyclic lactone produced by the bacterium Streptomyces hygroscopicus isolated from soil samples from Easter Island. Rapamycin is an antifungal agent with immunosuppressive properties. Rapamycin has antirejection properties without the side effects associated with other antirejection agents. Rapamycin binds to a specific protein, Target of Rapamycin (TOR). TOR is serine/threonine kinase. TOR (mTOR) forms two major complexes: mTORC1,and mTORC2. The mTORC1 consists of mTOR, Raptor, mLST8, FKBP38, PRAS40, and Deptor, and through specific binding of rapamycin to FKBP12, rapamycin inhibits the activity of mTORC1 leading to a decrease in protein synthesis, increased autophagy and inhibition of cell growth.
| | in vivo | Rapamycin (2.0 mg/kg; intraperitoneal injection; every other day; 28 days) alone has a moderate inhibitory effect. However, the combination of Metformin and Rapamycin exerts a significantly increased inhibition of tumor growth compared with the control group, the Rapamycin monotherapy group and the Metformin monotherapy group. | | Indications and Usage | Rapamycin (Rapa, or Sirolimus) is a new form of macrolide immunosuppressive agent. It is a white solid crystal. Its melting point is 183-185℃ and it is lipophilic. It is soluble in methanol, ethanol, acetone, chloroform, and other organic solvents, very slightly soluble in water, and almost insoluble in ether. It was first discovered in 1975 on the Chilean Easter Island as a secondary metabolite secreted by soil Streptomyces, and its chemical structure is that of a three-polyene macrolide compound.
Rapamycin is a new form of immunosuppressive agent with good curative effects, low toxicity, and no nephrotoxicity. It can be used to maintain the immunity of transplant organs (especially in kidney transplants) to alleviate immunological rejections after organ transplant surgeries. The latest research has shown that Rapamycin can also be used to treat Alzheimer’s. When used on afflicted lab rats, it had a memory-restoring effect. Rapamycin oral tablets can be taken with grapefruit juice to treat melanoma (a type of benign tumor common among Western populations), dramatically increase other chemotherapy drugs’ anticancer effects, and extend patient survival. Rapamycin is a mammalian target of rapamycin (mTOR) targeting inhibitor, which can treat tumors that are related to this pathway including kidney cancer, lymphoma, lung cancer, liver cancer, breast cancer, neuroendocrine carcinoma, gastric cancer, etc. Its curative effects are especially strong for the rare diseases LAM (lymphangiomyomatosis) and TSC (tuberous sclerosis).
| | Mechanisms of Action | Rapamycin is a type of macrolide antibiotic and has a similar structure to Prograf (FK506), but has very different immunosuppressing mechanisms. FK506 suppresses T lymph cells from proliferating from G0 to G1 stage, while RAPA uses different cytokine receptors to block signal transduction, thus preventing T lymph cells and other cells from proliferating from G1 to S stage. Compared to Prograf, Rapamycin can block the signal transduction pathways of the calcium dependency and calcium non-dependency of T lymph cells and B lymph cells.
| | Application | As an mTOR inhibitor, sirolimus has a broad spectrum of activity that has demonstrated the ability to inhibit inflammation, proliferation, angiogenesis, fibrosis, and hyperpermeability. Sirolimus currently has multiple uses in the prevention of rejection in organ transplantation and, recently, in the treatment of advanced renal cell carcinoma. Sirolimus-eluting cardiac stents have been shown to limit the rate of overgrowth of tissue and thus prevent coronary restenosis. Early studies suggest that it may be an effective agent for controlling severe uveitis and that it may also have a role in treating agerelated macular degeneration.
| | Adverse reactions | Rapamycin has similar side effects to those of Prograf. Many clinical trials have found that its side effects are dose dependent and reversible. A treatment dosage of Rapamycin has not been found to lead to any significant nephrotoxicity or gingival hyperplasia. Its main toxic side effects include: headache, nausea, dizziness, nosebleed, and join pain. Laboratory inspections for anomalies found: thrombocytopenia, decreased white blood cells, decreased hemoglobin, hypertriglyceridemia, hypercholesterolemia, hyperglycemia, increased liver enzymes (SGOT, SGPT), increased lactate dehydrogenase, hypokalemia, hypokalemia, etc. Rapamycin may lead to puffiness of eyelids and lead to lowered plasma phosphate levels. Similar to other immunosuppressants, Rapamycin may also increase the chance of infection, with reports of increased tendency towards pneumonia.
| | Binding Mode | The FKBP12–rapamycin binary complex shows
five hydrogen bonds within the binding site of FKBP12 domain of mTOR: (1) C40-OH to the amide
carbonyl of GIn53; (2) C28-OH to the amide carbonyl
of Glu54; (3) C1 carbonyl oxygen to the amide NH of
lle56; (4) C10-OH to the carboxylic acid side chain of
Asp37; and (5) C8 carbonyl oxygen to the tyrosine
phenol hydroxyl of Tyr82 (Figs.1,2).
  | | Description | Rapamycin is a member of the macrolide immunosuppressant family and a FRAP inhibitor. Rapamycin exhibits binding and inhibitory actions to the FK506 binding protein (FKBP5) proline rotamase via simultaneous binding by FKBP12 and FRAP. FRAP (RAFT1) proteins exhibit homology to PI 4- and PI 3-kinases, which have PI 4-kinase and autophosphorylating activities. The rapamycin/FKBP complex does not inhibit the FRAP PI 4-kinase activity, but does inhibit FRAP autophosphorylation. Rapamycin is unique in its ability to inhibit lymphokine induced cell proliferation at the G1 and S phase as well as an irreversible cellular arrest at the G1 phase in S. cerevisiae cells. Rapamycin also exhibits selective signal blocking leading to the activation of p70/85 S6 kinase, which is potentially due to the inhibition of FRAP autophosphorylation or protein kinase activity. Rapamycin also exhibits Angiogenesis inhibition, possibly through the inhibition of the Akt pathway. Rapamycin is an inhibitor of mTOR. Rapamycin is also known as Rapamune, 23,27-Epoxy-3H-pyrido[2,1-c] oxaazacyclohentriacontine, AY 22989, and mTOR Inhibitor I. | | Chemical Properties | White to Off-White Solid | | Originator | Rapamune,Wyeth Laboratories,UK | | Occurrence | Rapamycin was first discovered in 1972 in the soil of Easter Island produced by a bacterium called Streptomyces hygroscopicus. It takes its name from Rapa Nui, the indigenous name for the island. It is known clinically as sirolimus or Rapamune. | | Characteristics | Class: serine/threonine;
Treatment: organ rejection, LAM;
Other name: Sirolimus, AY-22989;
Oral bioavailability = 14%;
Elimination half-life = 63 h;
Protein binding = 92% | | Uses | Rapamycin is a triene macrolide discovered in 1995 as a metabolite of Streptomyces hygroscopicus found in a soil obtained on Rapi Nui (Easter Island). Rapamycin displayed potent and selective antifungal activity, notably against Candida albicans. Interest in the metabolite waned until the structural relationship to the potent immunosuppressant fujimycin (Antibiotic FK506) was recognised in the mid-1980s. This recognition led to the re-discovery of rapamycin as a highly selective antitumour and immunosuppressant. Rapamycin inhibits the activity of the protein, mTOR (mammalian target of rapamycin) which functions in a signalling pathway to promote tumour growth. Rapamycin binds to a receptor protein (FKBP12). The rapamycin/FKB12 complex then binds to mTOR and prevents interaction of mTOR with target proteins in this signalling pathway. | | Uses | Rapamycin is a triene macrolide discovered in 1974 as a metabolite of Streptomyces hygroscopicus found in a soil obtained on Rapa Nui (Easter Island). Rapamycin displayed potent and selective antifungal activity, notably against Candida albicans. Interest in the metabolite waned until the structural relationship to the potent immunosuppressant fujimycin (Antibiotic FK506) was recognised in the mid-1980s. This recognition led to the re-discovery of rapamycin as a highly selective antitumor and immunosuppressant. Rapamycin inhibits the activity of the protein, mTOR (mammalian target of rapamycin) which functions in a signalling pathway to promote tumor growth. Rapamycin binds to a receptor protein (FKBP12). The rapamycin/FKB12 complex then binds to mTOR and prevents interaction of mTOR with target proteins in this signalling pathway. | | Uses | Labelled Rapamycin. A triene macrolide antibiotic isolated from Streptomyces hygroscopicus. Name derived from the native word for Easter Island, Rapa?Nui. Used as an immunosuppressant; antirestenotic. This compound contains aproximately 2% d0.;Labeled Sir | | Uses | Rapamycin is an immunosuppressant that is used primarily to prevent the rejection of organ and bone marrow transplant. It was first described as a potent inhibitor of IL-2 activation of lymphocytes (IC50 = 5 pM). It is now known that rapamycin specifically interacts with the cytosolic FK-binding protein 12 (FKBP12) to form a complex which inhibits the mammalian target of rapamycin (mTOR) pathway by directly binding to mTOR Complex 1 (mTORC1). Rapamycin and other inhibitors of mTORC1 signaling show potential in treating cancer, adipogenesis, diabetes, tuberous sclerosis, and cardiovascular disease.[Cayman Chemical] | | Indications | Sirolimus (Rapamune) is structurally related to
tacrolimus. It is approved for use as an adjunctive agent
in combination with cyclosporine for prevention of
acute renal allograft rejection. It blocks IL-2-dependent
T-cell proliferation by inhibiting a cytoplasmic serine–
threonine kinase. This mechanism of action is different
from those of tacrolimus and cyclosporine. This allows
sirolimus to augment the immunosuppressive effects of
these drugs. | | Indications | Mechanistic target of rapamycin (mTOR) is a serine/threonine-specific protein kinase in the PI3/PI4-kinase family. mTOR was named after the natural macrolide rapamycin, also known as sirolimus, which was isolated from a soil sample from Easter Island in the 1970s and later evaluated as an immunosuppressive agent. The anticancer activity of rapamycin was discovered in the 1980s, although the mechanismof action and the identification of the rapamycin target, mTOR, were not elucidated until the 1990s. Rapamycin and its macrocyclic analogues, such as temsirolimus (Torisel(R), Wyeth/Pfizer) and everolimus (Afinitor(R), Novartis), are grouped as “rapalogs” that constitute the first-generation mTOR inhibitors.
Rapamycin was approved by the US FDA in 1999 as an immunosuppressive agent to prevent organ rejection in patients receiving kidney transplants. Although a large number of clinical studies have been performed to evaluate the anticancer activities of sirolimus in different types of cancers, such as invasive bladder cancer, breast cancer, and leukemia, most studies show limited efficacy. Outside oncological indications, sirolimus was approved by FDA for the treatment of a rare progressive lung disease lymphangioleiomyomatosis in 2015. Temsirolimus was approved for the treatment of advanced RCC. Everolimus was approved in the EU for the prevention of organ rejection in heart and kidney transplant recipients before FDA approved it in 2009 for the treatment of advanced RCC resistant to sunitinib or sorafenib and for the treatment of advanced or metastatic gastrointestinal and lung tumors in 2016. Additionally, rapamycin and rapalogs are being investigated as antiaging therapeutics or for the treatment of age-related diseases. Studies have revealed that mTOR activity can be retained under hypoxic conditions via mutations in the PI3K pathway, leading to increased translation and hypoxic gene expression and tumor progression. | | Definition | ChEBI: A macrolide isolated from Streptomyces hygroscopicus consisting of a 29-membered ring containing 4 trans double bonds, three of which are conjugated. It is an antibiotic, immunosupressive and antineoplastic agent. | | Manufacturing Process | Streptomyces hygroscopicus NRRL 5491 was grown and maintained on
oatmeal-tomato paste agar slants (T. G. Pridham et al.; Antibiotic Annual
1956-1957, Medical Encyclopedia Inc., New York, p. 947) and in Roux bottles
containing the same medium. Good growth was obtained after 7 days of
incubation at 28°C. Spores from one Roux bottle were washed off and
suspended into 50 ml of sterile distilled water. This suspension was used to
inoculate the first stage inoculum.The first-stage inoculum medium consisted of Emerson broth [R. L. Emerson
et al., J. Bacteriol, 52, 357 (1946)] 0.4% peptone, 0.4% sodium chloride,
0.25% yeast extract and 1% glucose; pH 7.0; flasks containing the above
medium were inoculated with 1 % of the spore suspension described above.
The inoculated flasks were incubated for 30 hours at 28°C on a reciprocating
shaker set at 65 r.p.m. (4 inch stroke).
Production stage
The production stage was run in 250-liter New Brunswick fermenters Model F-
250, equipped with automatic antifoam addition system and pH recordercontroller.
The fermenters were charged with 160 L of an aqueous production
medium consisting of the following constituents: 1.0% soluble starch; 0.5%
(NH4)2SO4; 0.5% K2HPO4; 1.5% glucose (Cerelose); 0.025% MgSO4; 0.005%
ZnSO4; 0.001% MnSO4; 0.002% FeSO47H2O; 0.2% CaCO3; 0.5% "Blackstrap"
molasses; 0.5% hydrolyzed casein; 0.2% lard oil; pH 7.1 to 7.3 of first stage
inoculum. Incubation temperature: 28°C; aeration: 0.5 vol/vol/min; agitation:
250 r.p.m. The fermenters were sterilized at 121°C for 45 min, cooled and
inoculated with one flask inoculum).
A titre of ca. 20 μg/ml, determined by microbiological assay on agar plates
seeded with Candida albicans, was reached in 5 days. The fermentation was
stopped. The fermentation mixture was extracted twice with 1 v/v of nbutanol.
The combined butanol extracts were washed with 1 v/v of water,
dried with anhydrous sodium sulfate and evaporated to dryness under reduced
pressure to yield a residue. The oily residue was extracted 3 times with 2 L of
methanol. The combined methanol extracts were passed through
diatomaceous earth (Celite) and evaporated to dryness to yield an oily residue
containing crude Rapamycin. | | Brand name | Rapamune (Wyeth). | | Therapeutic Function | Immunosuppressive, Antifungal | | General Description | Rapamycin is an anti-fungal antibiotic isolated from Streptomyces hygroscopicus. This antibiotic is active against all strains of Candida albicans. It blocks the signal transduction pathways required for the activation of T-helper cells. | | Biological Activity | Antifungal and immunosuppressant. Specific inhibitor of mTOR (mammalian target of Rapamycin). Complexes with FKBP-12 and binds mTOR inhibiting its activity. Inhibits interleukin-2-induced phosphorylation and activation of p70 S6 kinase. | | Biochem/physiol Actions | Rapamycin is a macrocyclic triene antibiotic possessing potent immunosuppressant and anticancer activity. It forms a complex with FKBP12 that binds to and inhibits the molecular target of rapamycin (mTOR). mTOR is a member of the phosphoinositide kinase-related kinase (PIKK) family that enhances cellular proliferation via the phosphoinositol 3-kinase/Akt signaling pathway. Inhibition of this pathway by rapamycin blocks downstream elements that result in cell cycle arrest in G1. The effectors of mTOR action include 4EBP1 and S6K1. | | Clinical Use | Immunosuppressant:
Prophylaxis of transplant allograft rejection | | in vitro | Rapamycin (12.5-100 nM; 24 hours) treatment exerts modest inhibitory effect on lung cancer cell proliferation in a dose-dependent manner in all cell lines (A549, SPC-A-1, 95D and NCI-H446 cells) tested, achieving about 30-40% reduction in cell proliferation at 100 nM vs. ~10% reduction at 12.5 nM.
Lung cancer cell line 95D cells are exposed to Rapamycin (10 nM, 20 nM) and RP-56976 (1 nM, 10 nM) alone or in combination (Rapamycin 20 nM+ RP-56976 10 nM). After 24 hours exposure to Rapamycin or RP-56976 alone does not significantly alter the level of expression or phosphorylation of ERK1/2, whereas cells treated with the combination of Rapamycin with RP-56976 exhibit a marked reduction in the phosphorylation levels of ERK1/2. | | target | mTOR | | Drug interactions | Potentially hazardous interactions with other drugs
Antibacterials: concentration increased by
clarithromycin - avoid; concentration of both drugs
increased with erythromycin; concentration reduced
by rifampicin and rifabutin - avoid.
Antifungals: concentration increased by itraconazole,
fluconazole, ketoconazole, micafungin, miconazole,posaconazole and voriconazole - avoid with
itraconazole, ketoconazole and voriconazole.
Antivirals: concentration possibly increased by
atazanavir, boceprevir and lopinavir; concentration
of both drugs increased with telaprevir, reduce
dose of sirolimus; concomitant use with dasabuvir
plus ombitasvir/paritaprevir/ritonavir is not
recommended unless the benefits outweigh the risks,
if used together administer sirolimus 0.2 mg twice a
week (every 3 or 4 days on the same two days each
week). Sirolimus blood concentrations should be
monitored every 4-7 days until 3 consecutive trough
levels have shown stable concentrations of sirolimus.
Sirolimus dose and/or dosing frequency should be
adjusted as needed.
Calcium-channel blockers: concentration increased
by diltiazem; concentration of both drugs increased
with verapamil.
Ciclosporin: increased absorption of sirolimus -
give sirolimus 4 hours after ciclosporin; sirolimus
concentration increased; long-term concomitant
administration may be associated with deterioration
in renal function.
Cytotoxics: use crizotinib with caution.
Grapefruit juice: concentration of sirolimus increased
- avoid.
Mycophenolate: concomitant use of mycophenolate
and sirolimus increases plasma levels of both
sirolimus and mycophenolic acid. | | Metabolism | Sirolimus is metabolised by the cytochrome P450
isoenzyme CYP3A4. Metabolism occurs by
demethylation or hydroxylation, and the majority of a
dose is excreted via the faeces. | | storage | -20°C | | References | 1) Kay et al. (1991) Inhibition of T and B lymphocyte proliferation by rapamycin. Immunology, 72 544
2) Mita et al., (2003) The molecular target of rapamycin (mTOR) as a therapeutic target against cancer; Cancer Biol. Ther., 2(4 Suppl 1) S169
3) Lamming et al. ( 2012). Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity; Science, 335 1638
4) Sarkar et al. (2009), Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies; Cell Death and Differ., 16 46 |
| | Rapamycin Preparation Products And Raw materials |
|