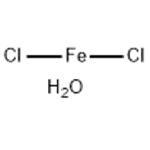
- Ferrous chloride tetrahydrate
-
- $6.00 / 1KG
-
2024-03-29
- CAS:13478-10-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
|
| | Ferrous chloride tetrahydrate Basic information |
| Product Name: | Ferrous chloride tetrahydrate | | Synonyms: | Ferrous Chloride, Tetrahydrate, GR;Iron(II) chloride tetrahydrate 99.99% trace Metals basis;Iron(II) chloride tetrahydrate puriss. p.a., >=99.0% (RT);Iron(II) chloride tetrahydrate ReagentPlus(R), 98%;Iron(II) chloride tetrahydrate Vetec(TM) reagent grade, 98%;Ferric(II) chloride tetrahydrate;IRON(II) CHLORIDE TETRAHYDRATE FOR ANALYSIS EMSURE;Ironchloridetetrahydrate | | CAS: | 13478-10-9 | | MF: | Cl2FeH2O | | MW: | 144.76 | | EINECS: | 603-870-5 | | Product Categories: | metal halide | | Mol File: | 13478-10-9.mol | 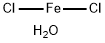 |
| | Ferrous chloride tetrahydrate Chemical Properties |
| Melting point | 105 °C | | Boiling point | 1026 °C | | density | 1.93 | | vapor pressure | 10 mm Hg ( 693 °C) | | storage temp. | Store at +15°C to +25°C. | | solubility | 1600g/l | | form | Solid | | color | Yellow to green | | Specific Gravity | 1.93 | | PH | 2.5 (100g/l, H2O, 20℃) | | Water Solubility | soluble | | Sensitive | Air Sensitive | | Merck | 14,4043 | | Exposure limits | ACGIH: TWA 1 mg/m3
NIOSH: TWA 1 mg/m3 | | Stability: | Air, moisture and light-sensitive. Incompatible with strong oxidizing agents, most common metals. | | InChIKey | WSSMOXHYUFMBLS-UHFFFAOYSA-L | | LogP | -1.380 (est) | | CAS DataBase Reference | 13478-10-9(CAS DataBase Reference) |
| Hazard Codes | Xn,C | | Risk Statements | 22-38-41-34 | | Safety Statements | 26-39-45-36/37/39 | | RIDADR | UN 3260 8/PG 3 | | WGK Germany | 1 | | RTECS | NO5600000 | | F | 3-23 | | TSCA | Yes | | HazardClass | 8 | | PackingGroup | III | | HS Code | 28273300 | | Toxicity | LD50 orally in Rabbit: 450 mg/kg |
| | Ferrous chloride tetrahydrate Usage And Synthesis |
| Chemical Properties | light green or blue-green crystals | | Uses | A variety of organically templated iron phosphates, of interest because of their rich crystal chemistry and their ability to absorb large molecules in their micropores,were prepared using this compound. | | Uses | Iron(II) chloride tetrahydrate is used as a reducing agent in metallurgy, in pharmaceutical preparations, as a mordant in dyeing and in sewage treatment. Ferrous chloride has a kind of niche application. During the laboratory synthesis of iron complexes, ferrous chloride acts as a reducing flocculating agent in waste water treatment, particularly for wastes containing chromate. It is the precursor to hydrated iron(III) oxides that are magnetic pigments. Ferrous chloride is employed as a reducing agent in many organic synthesis reactions. | | Definition | ChEBI: A hydrate that is the tetrahydrate form of iron dichloride. | | General Description | Iron(II) chloride tetrahydrate is a hydrated metallic halide. Crystal structure studies suggest that it crystallizes in monoclinic system having space group P21/c. | | Purification Methods | A 550mL round-bottomed Pyrex flask is connected, via a glass tube fitted with a medium porosity sintered-glass disc, to a similar flask. To 240g of FeCl2.4H2O in the first flask is added conductivity water (200mL), 38% HCl (10mL), and pure electrolytic iron (8-10g). A stream of purified N2 gas is passed through the assembly, escaping through a mercury trap. The salt is dissolved by heating which is continued until complete reduction has occurred. By inverting the apparatus and filtering (under N2 pressure) through the sintered glass disc, unreacted iron is removed. After cooling and crystallisation, the unit is again inverted, and the crystals of ferrous chloride are filtered free from mother liquor by applied N2 pressure. Partial drying by overnight evacuation at room temperature gives a mixed hydrate which, on further evacuation on a water bath at 80o, loses water of hydration and absorbed HCl (with vigorous effervescence) to give a white powder of FeCl2.2H2O (see below). [Gayer & Wootner J Am Chem Soc 78 3944 1956, (2H2O) Gayer & Woontner Inorg Synth V 179 1957.] |
| | Ferrous chloride tetrahydrate Preparation Products And Raw materials |
|