- Sodium Persulfate
-

- $8.00 / 1KG
-
2024-09-23
- CAS:7775-27-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 ton
- Sodium persulfate
-

- $520.00 / 1ton
-
2024-08-29
- CAS:7775-27-1
- Min. Order: 1ton
- Purity: 99%
- Supply Ability: 3000tons
- Sodium persulfate
-

- $0.00 / 1kg
-
2024-08-15
- CAS:7775-27-1
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 100tons
|
| Product Name: | Sodium persulfate | | Synonyms: | Formaldehyde Activator Solution, for Purpald Method;SODIUM PERSULFATE;Sodium Peroxodisulfate 〔Sodium Persulfate〕;SODIUM PEROXIDISULFATE FOR ANALYSIS EMSURE;FREE SAMPLE NCV SODIUM PERSULFATE;peroxydisulfuricacid([(ho)s(o)2]2o2),disodiumsalt;Peroxydisulfuricacid,disodiumsalt;persulfatedesodium | | CAS: | 7775-27-1 | | MF: | Na2O8S2 | | MW: | 238.1 | | EINECS: | 231-892-1 | | Product Categories: | Inorganics;Inorganic Chemicals | | Mol File: | 7775-27-1.mol | 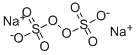 |
| | Sodium persulfate Chemical Properties |
| Melting point | 100 °C | | density | 2,4 g/cm3 | | vapor pressure | 0Pa at 25℃ | | storage temp. | Store at +15°C to +25°C. | | solubility | H2O: 1 M at 20 °C, clear, colorless | | form | Solid | | Specific Gravity | 2.4 | | color | White to yellow | | PH | 3.5-3.8 (100g/l, H2O, 20℃) | | Odor | Odorless | | PH Range | 2.5 - 4.0 | | Water Solubility | 550 g/L (20 ºC) | | Merck | 14,8656 | | Exposure limits | ACGIH: TWA 0.1 mg/m3 | | Stability: | Stability Unstable. Strong oxidizer. Contact with combustible material may cause fire. Incompatible with combustible material, strong reducing agents, strong bases, alcohols, aluminium, magnesium. Protect from moisture. | | InChIKey | CHQMHPLRPQMAMX-UHFFFAOYSA-L | | LogP | -1 at 20℃ | | CAS DataBase Reference | 7775-27-1(CAS DataBase Reference) | | EPA Substance Registry System | Sodium persulfate (7775-27-1) |
| Hazard Codes | O,Xn | | Risk Statements | 8-22-42/43-36/37/38 | | Safety Statements | 22-36/37-45-36/37/39-26-17 | | RIDADR | UN 1505 5.1/PG 3 | | WGK Germany | 1 | | RTECS | SE0525000 | | TSCA | Yes | | HS Code | 2833 40 00 | | HazardClass | 5.1 | | PackingGroup | III | | Toxicity | MLD in rabbits (mg/kg): 178 i.v. (DaVal) |
| | Sodium persulfate Usage And Synthesis |
| Physical and Chemical Properties | Sodium persulfate, also known as sodium peroxydisulfate is a white crystal or crystalline powder, odorless, tasteless. Formula is Na2S2O8, relative molecular mass is 238.13. Gradual decomposition at room temperature, heating or rapidly decompose in ethanol, decomposition to release oxygen and produce sodium pyrosulfate. Moisture and platinum black, silver, lead, iron, copper, magnesium, nickel, manganese and other metal ions or their alloys can promote the decomposition, it decomposes rapidly and emit hydrogen peroxide at high temperature (about 200 ℃). It is soluble in water (70.4 when 20 ℃).

Sodium persulfate has strong oxidizing. There is a strong irritation to the skin, prolonged contacting with the skin can cause allergies, should pay attention to it when operation. Rat oral LD50 is 895mg/kg. It should be Sealed storage. heat the ammonium persulfate and sodium hydroxide or sodium carbonate solution to remove carbon dioxide and ammonia to obtain sodium persulfate in the Laboratory.
| | Strong oxidants | With strong oxidizing, Sodium persulfate can be used as an g agent, which can oxidize Cr3 +, Mn2 + and so on to the corresponding compound of high oxidation state, when there is the presence of Ag +, which can promote the oxidation reaction. Due to its oxidizing properties, it can be used as a bleaching agent, metal surface treatment agent, chemical reagents, pharmaceutical raw materials, accelerator and initiator of battery and emulsion polymerization.
| | Uses | Sodium persulfate is used as a bleach, both standalone (particularly in hair cosmetics) and as a detergent component. It is a replacement for ammonium persulfate in etching mixtures for zinc and printed circuit boards, and is used for pickling of copper and some other metals. It is a source of free radicals, making it useful as an initiator for e.g. emulsion polymerization reactions and for accelerated curing of low formaldehyde adhesives. Sodium persulfate is also used as a soil conditioner and in manufacture of dyestuffs, modification of starch, bleach activator, desizing agent for oxidative desizing, etc.
For waste processing in the photographic industry, used as a soft metal surface corrosion agents of the printed circuit board and textile desizing agents, sulfur dyes colorformer.
| | Preparation method | 1. The electrolytic oxidation of the aqueous solution of ammonium sulfate is to obtain ammonium persulfate, and then metathesis reaction with sodium hydroxide, after the expulsion of the ammonia by-product, and then concentrated under reduced pressure, crystallization, drying, to obtain sodium sulfate.
(NH4) 2S2O8 + 2NaOH → Na2S2O8 + 2NH3 + 2H2O.
2. Dithionic acid can be prepared by electrolysis of cold sulfuric acid won, which reacts with alkali and then obtain sodium sulfate.
2HSO4--2e → H2S2O8
H2S2O8 + 2NaOH → Na2S2O8 + 2H2O.
| | storage | Sodium persulfate is a strong oxidizer and a severe irritant of skin, eyes, and respiratory system. It is almost non-hygroscopic and has particularly good ability to be stored for long time. It is easy and safe to handle. It is not combustible, but releases oxygen easily and assists combustion of other materials.
Conditions/ substances to avoid mixing persulfates with are: moisture, heat, flame, ignition sources, shock, friction, reducing agents, organic material, sodium peroxide, aluminum and powdered metals.
| | Chemical Properties | White, crystalline powder. Soluble in
water; decomposed by alcohol; decomposes in
moist air. | | Uses | Bleaching and oxidizing agent; promoter for emulsion polymerization reactions. | | Uses | Sodium peroxydisulfate is used as a radical initiator for emulsion polymerization reactions like acrylonitrile butadiene styrene, detergent component, soil conditioner and soil remediation. It is also used for curing of formaldehyde adhesives. It acts as a bleaching agent and in the production of dyestuffs. It finds application in zinc and printed circuit boards and considered to be a replacement for ammonium persulfate in etching mixtures. Further, it is used in the preparation of diapocynin from apocynin. It plays a role in the conversion of phenols to para-diphenols in alkaline solution and of arylamines to aminophenols. It is actively involved in the Elbs persulfate oxidation and the Boyland-Sims oxidation reactions as an oxidizing agent. | | Uses | Redi-Dri? is an innovative packaging product line that helps eliminate clumping material by adding extra packaging process steps without any chemical additives, anti-clumping agents, or hydrophobic compounds. Redi-Dri? offers a more convenient and safer way to handle the products. | | General Description | A white crystalline solid. Very irritating to skin and eyes. May be toxic by skin absorption. Used as a bleaching agent. | | Air & Water Reactions | Water soluble. Decomposes slowly in moist air. | | Reactivity Profile | Sodium persulfate is a strong oxidizing agent. Reacts with many combustible materials and reducing agents, often vigorously enough to start fires or cause explosions [Handling Chemicals Safely 1980 p. 855]. Decomposes gradually under ordinary conditions decomposition is promoted by moisture and heat [Merck]. Decomposed by alcohol and silver ions [Merck]. | | Hazard | By ingestion, strong irritant to tissue. | | Health Hazard | Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution. | | Fire Hazard | These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard. | | Flammability and Explosibility | Non flammable | | Safety Profile | Poison by intraperitoneal and intravenous routes. A powerful oxidizer; can cause fires. When heated to decomposition it emits toxic fumes of SOx and Na2O. See also SULFATES. |
| | Sodium persulfate Preparation Products And Raw materials |
|