|
|
| | Tranylcypromine Hemisulfate Basic information |
| Product Name: | Tranylcypromine Hemisulfate | | Synonyms: | tranylcyprominesulfate;tranylcyprominesulphate;Tranylcypromine Sulfate (125 mg);(1R,2S)-rel-2-Phenylcyclopropanamine Sulfate;TRANS-2-PHENYLCYCLOPROPYLAMINE HEMISULFATE;TRANS-2-PHENYLCYCLOPROPYLAMINE HEMISULFATE SALT;TRANYLCYPROMINE HEMISULFATE;TRANYLCYPROMINE HEMISULFATE SALT | | CAS: | 13492-01-8 | | MF: | C9H13NO4S | | MW: | 231.27 | | EINECS: | 236-807-1 | | Product Categories: | | | Mol File: | 13492-01-8.mol | 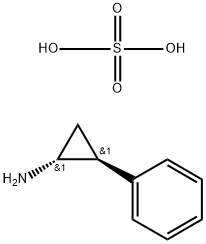 |
| | Tranylcypromine Hemisulfate Chemical Properties |
| storage temp. | 15-25°C | | solubility | Soluble in water; very slightly soluble in ethanol (96%) and in ether . | | form | White solid. | | color | White | | Stability: | Stable for 1 year from date of purchase as supplied. Solutions in distilled water may be stored at -20° for up to 3 months. | | InChIKey | BKPRVQDIOGQWTG-FKXFVUDVSA-N |
| Hazard Codes | T | | Risk Statements | 23/24/25 | | Safety Statements | 36/37/39-45 | | RIDADR | UN 2811 6.1/PG 2 | | WGK Germany | 3 | | RTECS | GZ2625000 | | HazardClass | 6.1(b) | | PackingGroup | III | | HS Code | 2921490002 |
| | Tranylcypromine Hemisulfate Usage And Synthesis |
| Description | Tranylcypromine (13492-01-8) is an irreversible and non-selective monoamine oxidase inhibitor.1,2?It has been shown to inhibit the histone demethylase BHC110/LSD1.3,4 | | Chemical Properties | A white or almost white, crystalline powder. | | Originator | Parnate,SKF,UK,1960 | | Uses | Monoamine oxidase inhibitor; antidepressant. | | Manufacturing Process | A solution containing 167 grams of stabilized styrene and 183 grams of ethyl
diazoacetate is cooled to 0°C and dropped into 83.5 grams of styrene with
stirring, in a dry nitrogen atmosphere, at 125° to 135°C. This produced the
ester ethyl 2-phenylcyclopropanecarboxylate.
A solution of the above ester (207.8 grams) and 64.5 grams of sodium
hydroxide in 80 cc of water and 600 cc of ethanol is refluxed for 9 hours. The
carboxylic acid of 2-phenylcyclopropane is liberated with 200 cc of
concentrated hydrochloric acid. The 2-phenylcyclopropanecarboxylic acid
contains 3 to 4 parts of the trans isomer to 1 part of the cis isomer. The acid
is recrystallized from hot water. The pure trans isomer comes out as
crystalline material (solid) while the cis isomer stays in solution.
A solution of 4.62 grams of 2-phenylcyclopropanecarboxylic acid in 15 cc of
dry benzene is refluxed with 4 cc of thionyl chloride for 5 hours, the volatile
liquids are removed and the residue once more distilled with benzene.
Fractionation of the residue yields the carbonyl chloride of 2-
phenylcyclopropane.
A mixture of 15 grams of technical sodium azide and 50 cc of dry toluene is
stirred and warmed and a solution of 10 grams of 2-
phenylcyclopropanecarbonyl chloride in 50 cc of dry toluene is added slowly.
Inorganic salts are filtered and washed well with dry benzene and the solvents
are removed under reduced pressure. The RCON3 compound produced
undergoes the Curtius rearrangement to RNCO + N2. The residual isocyanate
is a clear red oil of characteristic odor. It is cooled to 10°C and treated
cautiously with 100 cc of 35% hydrochloric acid whereupon RNCO + H2O gives
RNH2 + CO2.After most of the evolution of carbon dioxide has subsided the
mixture is refluxed for 13 hours, the cooled solution is diluted with 75 cc of
water and extracted with three 50 cc portions of ether. The acid solution is
evaporated under reduced pressure with occasional additions of toluene to
reduce foaming.
The almost dry residue is cooled to 0°C and made strongly alkaline with a
50% potassium hydroxide solution. The amine is extracted into several
portions of ether, dried over potassium hydroxide, the solvent removed, and
the base fractioned. Reaction of the base with a half-molar quantity of sulfuric
acid gives the sulfate. | | Therapeutic Function | Psychostimulant | | General Description | Tranylcypromine sulfate, (±)-trans-2-phenylcyclopropylaminesulfate (Parnate), was synthesized to be an amphetamineanalog (visualize the α-methyl of amphetaminecondensed onto the β-carbon atom).It does have someamphetamine-like properties, which may be why it has moreimmediate CNS-stimulant effects than agents that act byMAO inhibition alone. For MAO inhibition, there may betwo components to the action of this agent. One is thoughtto arise because tranylcypromine has structural features (thebasic nitrogen and the quasi-π character of the α- andβ-cyclopropane carbon atoms) that approximate the transitionstate in a route of metabolism of β-arylamines. As α-and β-hydrogen atoms are removed from the normal substrateof the enzyme, the quasi-π character develops over theα,β-carbon system. Duplication of the transition state permitsextremely strong, but reversible, attachment to the enzyme.Additionally, tranylcypromine is a mechanism-based inactivator.It is metabolized by MAO, with one electron of the nitrogenpair lost to flavin. This, in turn, produces homolyticfission of a carbon–carbon bond of cyclopropane, with oneelectron from the fission pairing with the remaining lone nitrogenelectron to generate an imine (protonated) and with theother residing on a methylene carbon. Thus, a free radical isformed that reacts to form a covalent bond with the enzymeor with reduced flavin to inactivate the enzyme. | | References | 1) Knoll?et al. (1980),?Monoamine oxidase inhibitors: Chemistry and Pharmacology; In, Sandler (ed) Enzyme inhibitors as drugs, MacMillan, London 151
2) Baker?et al. (1992),?Insights into the mechanisms of action of the MAO inhibitors phenelzine and tranylcypromine; a review, J.Psychiatry Neurosci.?17?206
3) Lee?et al. (2006),?Histone H3 lysine 4 demethylation is a target of nonselective antidepressive medications; Chemistry and Biology,?13?563
4) Schmidt and McCafferty (2007),?trans-2-Phenylcyclopropylamine is a Mechanism-Based Inactivator of the Histone Demethylase LSD1; Biochemistry,?46?4408 |
| | Tranylcypromine Hemisulfate Preparation Products And Raw materials |
|