- Hydroxychloroquine sulfate
-
- $8.00 / 1KG
-
2024-04-09
- CAS:747-36-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
Related articles - What is Hydroxychloroquine sulfate?
- Hydroxychloroquine sulfate (also known as hydroxychloroquine) is an antimalarial medicine approved in the United States for ei....
- Jul 22,2020
|
| | Hydroxychloroquine sulfate Basic information |
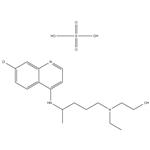
| Product Name: | Hydroxychloroquine sulfate | | Synonyms: | 2-((4-((7-chloro-4-quinolinyl)amino)pentyl)ethylamino)ethanolsulfate(1:1)(;2-((4-((7-Chloro-4-quinolinyl)amino)pentyl)ethylamino)ethanol sulfate (1:1) (salt);Ethanol, 2-((4-((7-chloro-4-quinolinyl)amino)pentyl)ethylamino)-, sulfate (1:1) (salt);Ethanol, 2-((4-((7-chloro-4-quinolyl)amino)pentyl)ethylamino)-, sulfate (1:1) (salt);Plaquinol;Hydroxychloroquine sulfate,98%;Hydroxychloroquin;Hydroxychloroquine Sulfate (200 mg) | | CAS: | 747-36-4 | | MF: | C18H28ClN3O5S | | MW: | 433.95 | | EINECS: | 212-019-3 | | Product Categories: | API;PLAQUENIL;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;1;747-36-4 | | Mol File: | 747-36-4.mol | 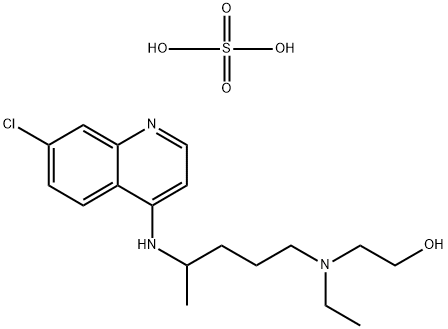 |
| | Hydroxychloroquine sulfate Chemical Properties |
| | Hydroxychloroquine sulfate Usage And Synthesis |
| Description | Hydroxychloroquine is an aminoquinoline with antimalarial, anti-inflammatory, and antiviral activities. It is active against the chloroquine-sensitive NF54 and D6 strains of P. falciparum (IC50s = 16.3 and 15 nM, respectively) but has decreased activity against chloroquine-resistant P. falciparum strains (IC50s = 422.7-1,735.3 nM). Hydroxychloroquine inhibits production of IL-22, IL-17A, and IL-6 induced by phorbol 12-myristate 13-acetate (TPA; ) and ionomycin in peripheral blood mononuclear cells (PBMCs) isolated from healthy individuals or patients with systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA). It inhibits accumulation of sequestosome-1 (SQSTM1) puncta, a marker of autophagy, in mouse embryonic fibroblasts (MEFs) in a concentration-dependent manner. Hydroxychloroquine reduces viral titers of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the culture supernatant of infected Vero E6 cells but does not reduce SARS-CoV-2 viral titers in an in vitro human airway epithelium model or the respiratory tract of infected cynomolgus macaques. Formulations containing hydroxychloroquine have been used in the prevention or treatment of malaria, as well as in the treatment of RA and SLE. | | Description | Hydroxychloroquine sulfate (also known as hydroxychloroquine) is an antimalarial medicine approved in the United States for either prevention or treatment of certain types of malaria, lupus erythematosus, and rheumatoid arthritis. It is sold under the brand name Plaquenil and it is also sold as a generic medicine. It is available in tablets of 155mg base (200mg salt).

Hydroxychloroquine sulfate has not been approved for the treatment of COVID-19. It has been used experimentally to treat certain people with COVID-19, including hospitalized patients. Hydroxychloroquine sulfate is being used to try and stop the COVID-19 virus from spreading inside your body. This may help you to get better.
Hydroxychloroquine sulfate is experimental because we do not know if it works for COVID-19. It is not approved by FDA for the treatment of COVID-19, but emergency use has been authorized for adults and adolescents who weigh 50 kg (110 pounds) or more and are hospitalized with COVID-19 if a clinical trial is not available or you are not able to participate in a clinical trial. There is limited information known about the safety and effectiveness (whether this will make you better) of using hydroxychloroquine sulfate for hospitalized patients with COVID-19. | | Chemical Properties | White Cyrstalline Solid | | Originator | Plaquenil,Winthrop,US,1956 | | Uses | antimalarial, lupus suppressant | | Uses | An antimalarial; antirheumatic; lupus erythematosus suppressant | | Uses | Hydroxychloroquine Sulfate can be used as a pharmaceutical secondary standard for the determination of the analyte in pharmaceutical formulations, and biological samples by various analytical techniques.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements. | | Manufacturing Process | A mixture of 323 grams of 1-chloro-4-pentanone, 480 grams of N-ethyl-N-2-
hydroxyethylamine and 400 grams of sodium chloride (to aid in subsequent
filtration) in 1.3 liters of xylene was heated with stirring on a steam bath for
two hours and then refluxed for three hours. After standing overnight, the
mixture was filtered and the filter cake washed with xylene. The filtrate was
fractionally distilled, yielding 207.3 grams of a fraction distilling at 89° to
90°C at 0.35 mm; nD25 = 1.4600. This fraction, 1-(N-ethyl-N-2-
hydroxyethylamino)-4-pentanone, was used in the next step of the synthesis.
A sample of the fraction was further purified by distillation through a column
and gave an analytically pure sample of 1-(N-ethyl-N-2-hydroxyethylamino)-
4-pentanone, boiling at 85° to 87°C at 0.4 mm.
The 1-(N-ethyl-N-2-hydroxyethylamino)-4-pentanone from above (284.2
grams) was dissolved in 300 grams of 28% ammoniacal methanol and
reduced catalytically with Raney nickel (at an initial pressure of 1,000 pounds)
at room temperature. After 24 hours the catalyst was filtered off and the
product distilled in vacuo through a column, yielding 254 grams of a fraction
distilling at 88.5° to 96°C at 0.3 mm and comprising mainly 5-(N-ethyl-N-2-
hydroxyethylamino)-2-pentylamine. An analytical sample of this fraction
distilled at 93°C at 0.6 mm.
A mixture of 90 grams of 4,7-dichloroquinoline, 90 grams of phenol, 1 gram
of potassium iodide and 132 grams of 5-(N-ethyl-N-2-hydroxyethylamino)-2-
pentylamine from above was heated with stirring for 13 hours at 125° to
130°C. Methanol (1.9 liters) was added and the the mixture was filtered with
charcoal. The filtrate was treated with 270 cc of a solution of 100 grams of
phosphoric acid in 300 cc of methanol. The walls of the flask containing the
filtrate were scratched with a glass rod and the mixture was allowed to stand
for two days. The solid was filtered off, washed with methanol and dried,
yielding 101 grams of crude 7-chloro-4-[5-(N-ethyl-N-2-hydroxyethylamino)-
2-pentyl]aminoquinoline diphosphate, MP 155° to 156°C.
Additional quinoline diphosphate was obtained as a gummy mass from the
filtrate by concentrating the latter to about half its volume and adding
acetone. The crude gummy diphosphate was dissolved in water, basified with
ammonium hydroxide and the resulting liberated basic quinoline extracted
with chloroform. After removal of the chloroform by distillation, the residue
was dissolved in ether and crystallization was induced by scratching the walls
of the flask with the glass rod. About 30 grams of the crude quinoline base,
melting at 77° to 82°C, separated. Recrystallization of this material from
ethylene dichloride or ethyl acetate yielded the purified 7-chloro-4-[5-(N�ethyl-N-2-hydroxyethylamino)2-pentyl] aminoquinoline, MP 89° to 91°C.
The base may then be dissolved in ethanol and precipitated as the sulfate by
reaction with an equimolar quantity of sulfuric acid. | | Therapeutic Function | Antimalarial | | General Description | Hydroxychloroquine Sulfate (Hydroxychloroquine Sulphate) is a salt of hydroxychloroquine (HCQ, Plaquenil), a 4-aminoquinoline based antiviral drug.
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. | | Biochem/physiol Actions | Hydroxychloroquine sulfate is an anti-inflammatory (via TNF), disease-modifying antirheumatic drug (DMARD), and a heme polymerase inhibitor. It is used in treating malaria. | | Pharmacology | Antimalarial action: Hydroxychloroquine binds to DNA, interfering with protein synthesis. It also inhibits DNA and RNA polymerases. It’s active against asexual erythrocytic forms of Plasmodium malariae, P. ovale, P. vivax, and many strains of P. falciparum.
Amebicidal action: Mechanism of action is unknown.
Anti-inflammatory action: Mechanism of action is unknown. Drug may antagonize histamine and serotonin and inhibit prostaglandin effects by inhibiting conversion of arachidonic acid to prostaglandin F2; it may also inhibit chemotaxis of polymorphonuclear leukocytes, macrophages, and eosinophils. | | Pharmacokinetics | Absorption: Absorbed readily and almost completely.
Distribution: Bound to plasma proteins. It’s concentrated in the liver, spleen, kidneys, heart, and brain and is strongly bound in melanin-containing cells.
Metabolism: Metabolized by the liver to desethylchloroquine and desethyl hydroxychloroquine.
Excretion: Most of an administered dose is excreted unchanged in urine. Drug and its metabolites are excreted slowly in urine; unabsorbed drug is excreted in feces. Small amounts of drug may be present in urine for months after it’s discontinued. Drug appears in breast milk. | | Clinical Use | Hydroxychloroquine sulfate is highly water soluble and exists in two different forms of different melting points. It is readily absorbed on oral administration, reaching peak plasma levels within 1 to 3 hours. It concentrates in organs such as the liver, spleen, kidneys, heart, lung, and brain, thereby prolonging elimination. Hydroxychloroquine is metabolized by N-dealkylation of the tertiary amines, followed by oxidative deamination of the resulting primary amine to the carboxylic acid derivative. In addition to possessing corneal and renal toxicity, hydroxychloroquine also may cause CNS, neuromuscular, GI, and hematological side effects. Hydroxychloroquine sulfate is indicated for the treatment of rheumatoid arthritis, lupus erythematosus, and malaria. | | Side effects | In addition to possessing corneal and renal toxicity, hydroxychloroquine also may
cause CNS, neuromuscular, GI, and hematological side effects. Hydroxychloroquine sulfate is indicated for the
treatment of rheumatoid arthritis, lupus erythematosus, and malaria | | Overdosage | Symptoms of drug overdose may appear within 30 minutes after ingestion and may include headache, drowsiness, visual changes, CV collapse, and seizures followed by respiratory and cardiac arrest.
Treatment is symptomatic. Empty stomach by emesis or lavage. After lavage, activated charcoal in an amount at least five times the estimated amount of drug ingested may be helpful if given within 30 minutes of ingestion.
Ultra-short-acting barbiturates may help control seizures. Intubation may become necessary. Peritoneal dialysis and exchange transfusions may also be useful. Forced fluids and acidification of the urine are helpful after the acute phase. | | Drug interactions | Anti-arrhythmics: increased risk of ventricular arrhythmias when hydroxychloroquine administered with amiodarone – avoid concomitant use
Digoxin: Hydroxychloroquine sulfate has been reported to increase plasma digoxin levels: serum digoxin levels should be closely monitored in patients receiving concomitant therapy.
Antibacterials: increased risk of ventricular arrhythmias when hydroxychloroquine administered with moxifloxacin – avoid concomitant use.
Ciclosporin: hydroxychloroquine increases plasma concentration of ciclosporin (increased risk of toxicity) .
Antacids: as with chloroquine, antacids may reduce absorption of hydroxychloroquine so it is advised that a 4 hour interval be observed between hydroxychloroquine and antacid dosaging.
Antidiabetic medicines: As hydroxychloroquine may enhance the effects of hypoglycaemic treatment, a decrease in doses of insulin or antidiabetic drugs may be required.
Tamoxifen: may enhance the adverse/toxic effect of Hydroxychloroquine. Specifically, concomitant use of tamoxifen and hydroxychloroquine may increase the risk of retinal toxicity. | | Metabolism | Hydroxychloroquine is metabolised to chloroquine,
which in turn is extensively metabolised in the
liver, mainly to monodesethylchloroquine with
smaller amounts of bisdesethylchloroquine
(didesethylchloroquinine) and other metabolites being
formed. Monodesethylchloroquine has been reported to
have some activity against Plasmodium falciparum.
Chloroquine and its metabolites are excreted in the urine,
with about half of a dose appearing as unchanged drug
and about 10% as the monodesethyl metabolite. | | storage | Store at +4°C |
| | Hydroxychloroquine sulfate Preparation Products And Raw materials |
|