|
|
| Product Name: | 1,2,3,4-TETRAHYDROISOQUINOLINE | | Synonyms: | 1,2,3,4-Tetrahydro-2-azanaphthalene;1,2,3,4-Tetrahydro-2-isoquinoline;1,2,3,4-tetrahydro-isoquinolin;LABOTEST-BB LTBB000776;AKOS BB-9293;1,2,3,4-TETRAHYDROISOQUINOLINE;1,2,3,4-Tetrahydro-#niso-quinoline;3,4-Dihydro-1H-isoquinoline. | | CAS: | 91-21-4 | | MF: | C9H11N | | MW: | 133.19 | | EINECS: | 202-050-0 | | Product Categories: | Bioactive Small Molecules;Building Blocks;Cell Biology;Chemical Synthesis;Heterocyclic Building Blocks;T;Quinoline&Isoquinoline;Building Blocks;Heterocyclic Building Blocks;Isoquinolines;bc0001 | | Mol File: | 91-21-4.mol | 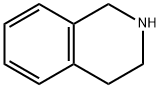 |
| | 1,2,3,4-TETRAHYDROISOQUINOLINE Chemical Properties |
| Melting point | −30 °C(lit.) | | Boiling point | 232-233 °C(lit.) | | density | 1.064 g/mL at 25 °C(lit.) | | vapor pressure | 3.33-97.9Pa at 20-58.5℃ | | refractive index | n20/D 1.568(lit.) | | Fp | 210 °F | | storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | solubility | 20g/l | | form | Liquid | | pka | 9.66±0.20(Predicted) | | color | Clear yellow to brown | | Water Solubility | Soluble in water at 20°C 20g/L. | | BRN | 116156 | | InChIKey | UWYZHKAOTLEWKK-UHFFFAOYSA-N | | CAS DataBase Reference | 91-21-4(CAS DataBase Reference) | | EPA Substance Registry System | Isoquinoline, 1,2,3,4-tetrahydro- (91-21-4) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 3 | | RTECS | NX4900000 | | Hazard Note | Irritant | | TSCA | Yes | | HS Code | 29334900 |
| | 1,2,3,4-TETRAHYDROISOQUINOLINE Usage And Synthesis |
| Synthesis | To a solution of the isoquinolinone (1.156 g, 9.90 mmol) and tert-butyl alcohol
(0.88 mL, 11.9 mmol) in THF (30 mL) at −78 °C was added liquid ammonia (about
280 mL). Lithium was added in small pieces until the blue coloration persisted, after
which the solution was stirred at −78 °C for 30 min. The blue coloration was dissipated
with piperlyne, 4-methoxybenzyl chloride (4.83 g, 31.00 mmol) in THF (5 mL)
was introduced by syringe, and the mixture was stirred for an additional 150 min at
−78 °C. Solid ammonium chloride was added and then the ammonia was allowed to
evaporate. The pale yellow residue was partitioned between CH2Cl2 (30 mL) and
water (40 mL). The layers were separated, and the aqueous layer was extracted
with CH2Cl2 (2 × 30 mL). The combined organic layers were washed with 10%
sodium thiosulfate solution (20 mL), dried over magnesium sulfate, and concentrated.
Flash chromatography (EtOAc:hexane, 2:1) on silica gave 2.21 g (75%) of the
tetrahydroisoquinolinone. 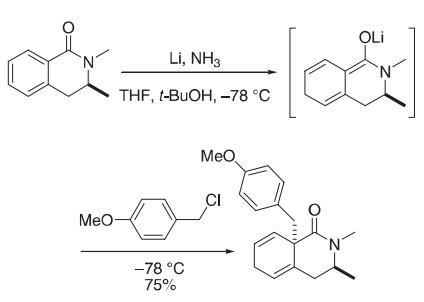
Reference: Schultz, A. G.; Guzi, T. J.; Larsson, E.; Rahm, R.; Thakker, K.
Bidlack, J. M. J. Org. Chem. 1998, 63, 7795–7804. | | Description | 1, 2, 3, 4-TETRAHYDROISOQUINOLINE belongs to tetrahydroisoquinoline compound, which is encountered in a number of drugs, tubocurarine, one of the quaternary ammonium muscle relaxants1-3. It is used as a reagent in the preparation of4-(1, 2, 4-oxadiazol-5-yl) piperidine-1-carboxamides as antiproliferative tubulin inhibitors1. It can be used for the synthesis of 1, 2, 3, 4-tetrahydroisoquinoline-3-carboxylic acid (Tic), which has strong applications in peptides and peptidomimetics design and discovery2. 1, 2, 3, 4-tetrahydroisoquinoline has been made into some derivatives with potential for prevention of parkinsonism4, cancer treatment5 and acting as Anticonvulsant Agents6.
| | Reference |
- https://www.alfa.com/en/catalog/L08143/
- https://www.ncbi.nlm.nih.gov/pubmed/21235510
- https://en.wikipedia.org/wiki/Tetrahydroisoquinoline
- Okuda, K, Y. Kotake, and S. Ohta. "Parkinsonism-preventing activity of 1-methyl-1, 2, 3, 4-tetrahydroisoquinoline derivatives in C57BL mouse in vivo. Biological & Pharmaceutical Bulletin 29.7(2006):1401-1403.
- Luca, Laura De, et al. "3D Pharmacophore Models for 1, 2, 3, 4‐Tetrahydroisoquinoline Derivatives Acting as Anticonvulsant Agents." Archiv Der Pharmazie 339.7(2006):388-400.
- Hatano, H, et al. "Tumor-specific cytotoxic activity of 1, 2, 3, 4-tetrahydroisoquinoline derivatives against human oral squamous cell carcinoma cell lines."Anticancer Research 29.8(2009):3079-3086.
| | Chemical Properties | clear yellow to brown liquid | | Uses | 1,2,3,4-Tetrahydroisoquinoline is used as a reagent in the preparation of 4-(1,2,4-oxadiazol-5-yl)piperidine-1-carboxamides as antiproliferative tubulin inhibitors. | | Definition | ChEBI: 1,2,3,4-tetrahydroisoquinoline is a member of isoquinolines. | | Synthesis Reference(s) | The Journal of Organic Chemistry, 40, p. 1191, 1975 DOI: 10.1021/jo00897a001
Tetrahedron Letters, 26, p. 4633, 1985 DOI: 10.1016/S0040-4039(00)98771-9 |
| | 1,2,3,4-TETRAHYDROISOQUINOLINE Preparation Products And Raw materials |
| Raw materials | Ethanol-->Sodium hydroxide-->Methanol-->Diethyl ether-->Acetic acid-->Chloroform-->Sodium sulfate-->Sodium bicarbonate-->Trifluoroacetic acid-->Ethyl formate-->Paraformaldehyde-->2-PhenylethylaMine-->Isoquinoline, 1,2-dihydro--->Isoquinoline,3,4-dihydro-, 2-oxide-->2-(4-nitrophenyl)sulfonyl-3,4-dihydro-1H-isoquinoline-->N-FORMYL-1,2,3,4-TETRAHYDROISOQUINOLINE-->Isoquinoline, 2-benzoyl-1,2,3,4-tetrahydro--->Isoquinoline, 1,2,3,4-tetrahydro-2-hydroxy- | | Preparation Products | 6-AMINO-2-N-BOC-1,2,3,4-TETRAHYDRO-ISOQUINOLINE |
|