- Boron tribromide
-

- $10.00 / 10g
-
2024-04-15
- CAS:10294-33-4
- Min. Order: 10g
- Purity: 99
- Supply Ability: 100ton
- Boron tribromide
-

- $100.00 / 1bag
-
2023-09-13
- CAS:10294-33-4
- Min. Order: 1bag
- Purity: 99
- Supply Ability: 5000
- Boron tribromide
-

- $120.00 / 1KG
-
2023-08-16
- CAS:10294-33-4
- Min. Order: 1KG
- Purity: >99%
- Supply Ability: 50000kg/Month
Related articles - Applications of Boron tribromide
- Boron tribromide, BBr3, is a colorless, fuming liquid compound containing boron and bromine. Commercial samples usually are am....
- Nov 11,2019
|
| | Boron tribromide Chemical Properties |
| Melting point | −46 °C(lit.) | | Boiling point | ~90 °C | | density | 2.60 g/mL at 20 °C(lit.) | | vapor density | 8.6 (vs air) | | vapor pressure | 40 mm Hg ( 14 °C) | | Fp | 30 °F | | refractive index | 1.4340 | | storage temp. | Poison room | | solubility | Miscible with ethanol and carbon tetrachloride. | | form | Solution | | Specific Gravity | 2.65 | | color | colorless | | Water Solubility | Reacts with water. | | Sensitive | Moisture Sensitive | | Merck | 14,1347 | | Dielectric constant | 2.6(0℃) | | Stability: | Stable, but reacts violently with water. | | InChIKey | ILAHWRKJUDSMFH-UHFFFAOYSA-N | | CAS DataBase Reference | 10294-33-4(CAS DataBase Reference) | | EPA Substance Registry System | Boron tribromide (10294-33-4) |
| | Boron tribromide Usage And Synthesis |
| General Description | Boron tribromide is commercially available and is a strong Lewis acid. It is an excellent demethylating or dealkylating agent for ethers, often in the production of pharmaceuticals.
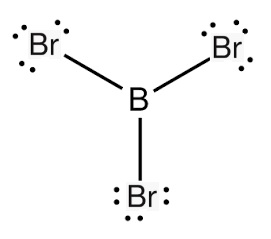
boron tribromide lewis structure
Additionally, it also finds applications in olefin polymerisation and in Friedel–Crafts chemistry as a Lewis acid catalyst. The electronics industry uses boron tribromide as a boron source in pre-deposition processes for doping in the manufacture of semiconductors. Boron tribromide is a colourless, fuming liquid compound containing boron and bromine. It is usually made by heating boron trioxide with carbon in the presence of bromine: this generates free boron that reacts vigorously with the bromine. Boron tribromide is used extensively in industries associated with pharmaceutical manufacturing, image processing, semiconductor doping, plasma etching, and photovoltaic manufacturing and as a reagent for different chemical processes.
| | Physical and Chemical Properties | Fuming colorless viscous liquid with a strong irritant, toxic. Melting point is-46 ℃, the boiling point is 91.3 ℃. It was dissolved in carbon tetrachloride. Easily decomposed by water, alcohol. light or thermal decomposition, heated to explode. It can react with the phosphorus, nitrogen, oxygen, sulfur, halogens, ammonia, alkali, phosphorus halides, phosphines, and many substituents of ammonia. A strong corrosive. Strong irritative to Skin, eye or mucous membrane. Approximate toxicity of hydrogen bromide. United States provides operating maximum allowable concentration of boron tribromide in environment Air is 1ppm (10mg/m3). It is obtained in laboratory by Aluminum tribromide reacting with boron trifluoride, then distillation. can be used as a source of doped semiconductor silicon, but also for the preparation of high purity boron and organic boride.
Other related chemical reactions involved by boron tribromide:
In hydroiodic, at 300~400 ℃ continuously fed in boron tribromide, obtained mixture of BIBr2 and BI2Br, BI3, and then separated by distillation, derived dibromo iodide boron.
Boron tribromide reacts with adamantine, generates 1-bromo-adamantane.
The above information is edited by the chemicalbook of Yan Yanyong.
| | Boron trichloride | Boron trifluoride, boron trichloride, boron tribromide and boron triiodide are four kinds of common boron halides, the last three kinds of halogenated boron can be made in the presence of carbonaceous reducing agent by the high-temperature oxidation reduction of halogens and diboron trioxide, the reaction equation is as follows: B2O3 + 3C + 3Cl2 = 2BCl3 + 3CO, boron trifluoride is obtained by interaction of hydrogen fluoride (fluorspar with concentrated sulfuric acid) and diboron trioxide. Boron halide are all covalent molecules, in the vapor state existing in a planar triangles single molecule. Boron atoms using sp2 hybrid orbitals, p orbital of boron atoms filled of electron in the vertical plane perpendicular to the empty p orbital plane of a halogen atom can form large π bond π64. Experimental results show that the measured bond length (such as B-Cl bond length is 173pm) is shorter than a single bond (single bond B-Cl bond length is 187pm), indicating the presence of large π bond. The melting points of all these four kinds of halides are low, boron trifluoride is the lowest, and the boiling point increases with the increase of atomic number of halogen, indicating four kinds of halide are covalent halide molecules, intermolecular attraction is van der Waals forces. In 4 kinds of halides, stability is declined from boron trifluoride in turn to boron triiodide. Boron halides are easily hydrolyzed to produce boric acid.
| | Toxicity | Boron tribromide has a strong stimulating effect on human tissue, and its vapor is highly toxic, strong corrosive. Wear masks, gloves and protective clothing during operation. Steam inhalation is strictly prohibited. Immediately sent to hospital for treatment after poisoning.
| | Uses | as the major chemical raw material of the production of caustic soda, soda ash, widely used in alkali industry.
As a semiconductor silicon doping source, it can be used for preparation of high purity boron and organic boron compound.
Trona is mainly used for making soda ash, caustic soda, soda, and other products. Soda ash is an important industrial raw material, widely used in glass, chemicals, light industry, textile, dyeing, metallurgy, petroleum processing, pharmaceuticals, food and so on. Caustic soda is mainly used for rayon, paper, dyes, soap, plastics, pharmaceuticals, agricultural chemicals and so on. Baking soda is mainly used in food, plastics, rubber, pharmaceutical, printing and dyeing, tanning, soaking seeds and other areas.
As a dopant materials of semiconductor, catalyst, intermediate and brominated agent of organic synthesis, It is a raw material for producing high purity boron and other organic boron compound.
Used in organic synthesis and preparation for high purity boron.
As catalyst, intermediates and Brominating agents for organic synthesis, raw materials for manufacturing high purity boron and other organic boron compound.
| | Production method | Direct synthesis is putting the dried powder of boron into the reaction tube of a tubular reactor, to make the reaction can be carried out sufficiently, a certain amount of filler should be placed in the reaction tube, the filler material is the same as the inner wall of the reaction tube. The reaction tube was heated to 850 ℃, also bromine in the bromine vessel was heated to a boil, then poured into reaction tube. The boron tribromide liquid produced with activated carbon, zinc and aluminum scrap together in debromination vessel heated to reflux until boron bromide produced is a colorless, and then by crude distillation, distillation, obtained completely colorless bromide boron products. 2B + 3Br2 → 2BBr3
Salt Lake alkaline mineral general uses open-pit mining, ancient alkaline mineral general uses underground dissolution mining law. Mining process
1. open-pit mining 2. underground dissolution method
| | Description | Boron tribromide is a colorless, fumingliquid. Molecular weight=250.57. Boiling point=90℃;Freezing/Melting point=-46℃; Vapor pressure=53 hPaat 14℃. Hazard Identification (based on NFPA-704 MRating System): Health 3, Flammability 0, Reactivity 2.Soluble in water; dangerous reaction. | | Chemical Properties | clear to amber liquid with a pungent odour | | Chemical Properties | Boron tribromide is a colorless, fuming liquid. | | Uses | Catalyst in manufacture of diborane,
ultrahigh-purity boron, and semiconductors | | Uses | Boron Tribromide is used as a reagent in the synthesis of 8-hydroxyquinolato compounds used as electron transport materials in EL devices. It is also used in the demethylation of aryl methyl ethers by
boron tribromide. | | Uses | manufacture of diborane; ultra high purity boron. Reagent for cleavage of ethers, amines, thiols; addition of allenes and alkynes. | | Application | The primary use of
boron tribromide is as an initiator for the
polymerization of olefins and as a catalyst in
other organic reactions. It is also used in the
electronics industry as a source of bromine for
ion implantation in semiconductors, and for
plasma etching in semiconductor device
manufacturing. | | General Description | A colorless, fuming liquid with a pungent odor. Boiling point 194°F. Freezing point -51°F. Very toxic by inhalation. Corrosive to metals and tissue. | | Reactivity Profile | Boron tribromide strongly attacks wood and rubber with generation of flammable hydrogen gas. Reacts exothermically and violently with water. Mixing tungsten trioxide and Boron tribromide caused an explosion when the reaction was not cooled in an ice bath. | | Hazard | Corrosive to tissue. May explode when
heated. Upper respiratory tract irritant. | | Health Hazard | Inhalation causes severe irritation of mucous membranes. Ingestion causes burns of mouth and stomach. Contact with eyes or skin causes severe burns. | | Fire Hazard | Special Hazards of Combustion Products: Toxic fumes of the chemical or hydrogen bromide may form in fires. | | Potential Exposure | Boron tribromide is highly toxic and
corrosive, it is used as a catalyst in organic synthesis, making
diborane, high purity boron, and semiconductors. | | First aid | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions,including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. If victim is conscious,administer water or milk. Do not induce vomiting.Medical observation is recommended for 24� 48 h after breathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroidspray. | | storage | (1) Color Code—White: Corrosive or ContactHazard; Store separately in a corrosion-resistant location.(2) Color Code—Blue: Health Hazard/Poison: Store in asecure poison location. Before entering confined spacewhere boron tribromide may be present, check to makesure that an explosive concentration does not exist.Store in airtight, unbreakable containers in a cool wellventilated area away from water, steam, potassium,sodium, alcohol, and other incompatible materials. Metalcontainers involving the transfer of this chemical should begrounded and bonded. Where possible, automatically pumpliquid from drums or other storage containers to processcontainers. Drums must be equipped with self-closingvalves, pressure vacuum bungs, and flame arresters. Useonly nonsparking tools and equipment, especially whenopening and closing containers of this chemical. Sourcesof ignition, such as smoking and open flames, are prohibited where this chemical is used, handled, or stored in amanner that could create a potential fire or explosionhazard. | | Shipping | UN2692 Boron tribromide, Hazard class: 8;
Labels: 8—Corrosive materials, 6.1—Poison Inhalation
Hazard, Inhalation Hazard Zone B. | | Incompatibilities | Reacts violently and explosively with
water, steam, or alcohols, forming toxic, corrosive, and
potentially explosive hydrogen bromide gas. Mixtures with
potassium or sodium can explode on impact. Incompatible
with oxidizers, strong bases. Attacks some metals, rubbers,
and plastics. |
| | Boron tribromide Preparation Products And Raw materials |
| Raw materials | Government regulation-->Boron | | Preparation Products | Sodium hydroxide-->Sodium carbonate-->Sodium bicarbonate-->(S)-(-)-7,7'-BIS[DI(3,5-DIMETHYLPHENYL)PHOSPHINO]-2,2',3,3'-TETRAHYDRO-1,1'-SPIROBIINDANE-->(R)-7,7'-BIS(DIPHENYLPHOSPHINO)-1,1'-SPIROBIINDANE-->Sodium silicate-->(S)-1,1'-SPIROBIINDANE-7,7'-DIOL-->(11AR)-(+)-10,11,12,13-TETRAHYDRODIINDENO[7,1-DE:1',7'-FG][1,3,2]DIOXAPHOSPHOCIN-5-BIS(R)-1PHENYLETHYL]AMINE-->2,6-Difluoro-4-hydroxybenzonitrile-->(11AR)-(+)-10,11,12,13-TETRAHYDRODIINDENO[7,1-DE:1',7'-FG][1,3,2]DIOXAPHOSPHOCIN-5-PHENOXY-->(11AR)-(+)-10,11,12,13-TETRAHYDRODIINDENO[7,1-DE:1',7'-FG][1,3,2]DIOXAPHOSPHOCIN-5-DIMETHYLAMINE-->1,1'-SPIROBIINDANE-7,7'-DIOL-->PYRIDAZINE-3,4-DIAMINE-->NU1025-->H-GLY-AMC HBR-->methyl 2-(7-hydroxybenzofuran-3-yl)acetate-->Bis(pinacolato)diboron-->2-[3-[Bis(1-methylethyl)amino]-1-phenylpropyl]-4-methylphenol-->3-FLUORO-5-METHOXY-PHENOL-->5-Hydroxypyrimidine-->4-(1,2,3-THIADIAZOL-4-YL)PHENOL-->5-FLUORO-4-HYDROXY-2-METHOXYBENZONITRILE-->TRIS(4-HYDROXYPHENYL)PHOSPHINE OXIDE-->4-BROMOQUINOLIN-2(1H)-ONE-->TRIS(PENTAFLUOROPHENYL)BORANE |
|