- 1,2,4-Trichlorobenzene
-

- $0.00 / 5tons
-
2024-09-19
- CAS:120-82-1
- Min. Order: 5tons
- Purity: 99.8%
- Supply Ability: 20000
- 1,2,4-Trichlorobenzene
-

- $10.00 / 1KG
-
2024-09-19
- CAS:120-82-1
- Min. Order: 1KG
- Purity: 99.%
- Supply Ability: 10 ton
- 1,2,4-Trichlorobenzene
-

- $50.00 / 1kg
-
2024-08-15
- CAS:120-82-1
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 10 tons
|
| | 1,2,4-Trichlorobenzene Basic information |
| Product Name: | 1,2,4-Trichlorobenzene | | Synonyms: | trojchlorobenzen(polish);UNSYM-TRICHLOROBENZENE;Trichlorobenzene, 1,2,4-? Trichlorobenzol;1,2,4-TRICHLOROBENZENE PESTANAL;1,2,4-TRICHLOROBENZENE, 99+%, &;1,2,4-TRICHLOROBENZENE, REAGENTPLUS, >=99% (POLY COATED BOTTLE);1,2,4-TRICHLOROBENZENE, 5000MG, NEAT;1,2,4-TRICHLOROBENZENE, REAGENTPLUS, >=99% | | CAS: | 120-82-1 | | MF: | C6H3Cl3 | | MW: | 181.45 | | EINECS: | 204-428-0 | | Product Categories: | Alpha Sort;Analytical Standards;Chemical Class;Halogenated;TP - TZ;T-ZAlphabetic;Amber Glass Bottles;Carbon Steel Cans with NPT Threads;ReagentPlus(R) Solvent Grade Products;ReagentPlus(R)Semi-Bulk Solvents;ReagentPlus(R)Solvents;Solvent Bottles;Anhydrous Grade SolventsSolvents;Solvents;Sure/Seal? Bottles;Pesticides&Metabolites;Q-ZAlphabetic;ACS and Reagent Grade Solvents;Amber Glass Bottles;Carbon Steel Cans with NPT Threads;ReagentPlus;ReagentPlus Solvent Grade Products;Semi-Bulk Solvents;Organics;Analytical Chemistry;Standard Solution of Volatile Organic Compounds for Water & Soil Analysis;Standard Solutions (VOC);Spectrophotometric Grade Solvents;Spectrophotometric GradeSolvents;AromaticsChemical Class;ChloroVolatiles/ Semivolatiles;Solvent Bottles;Solvent by Application;Solvent Packaging Options;Anhydrous Solvents;Sure/Seal Bottles | | Mol File: | 120-82-1.mol | 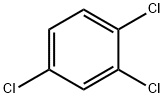 |
| | 1,2,4-Trichlorobenzene Chemical Properties |
| Melting point | 16 °C(lit.) | | Boiling point | 214 °C(lit.) | | density | 1.454 g/mL at 25 °C(lit.) | | vapor density | >6 (vs air) | | vapor pressure | 1 mm Hg ( 40 °C) | | refractive index | n20/D 1.571(lit.) | | Fp | >230 °F | | storage temp. | 2-8°C | | solubility | water: insoluble | | form | Liquid | | color | Clear | | Odor | Characteristic aromatic odor | | explosive limit | 6.6%, 150°F | | Water Solubility | INSOLUBLE | | λmax | λ: 308 nm Amax: 1.00
λ: 310 nm Amax: 0.50
λ: 350 nm Amax: 0.05
λ: 375-400 nm Amax: 0.01 | | Merck | 14,9631 | | BRN | 956819 | | Henry's Law Constant | 0.997 at 20.0 °C (wetted-wall column, ten Hulscher et al., 1992) 1.24, 2.27, 2.58, 3.06, and 3.90 at 2.0, 6.0, 10.0, 18.0, and 25.0 °C, respectively (EPICS-SPME,
Dewulf et al., 1999) | | Exposure limits | NIOSH REL: TWA ceiling 5 ppm (40 mg/m3); ACGIH TLV: ceiling 5 ppm
(adopted). | | Dielectric constant | 2.2400000000000002 | | Stability: | Stable. Incompatible with strong oxidizing agents. Combustible. | | LogP | 4.28 | | CAS DataBase Reference | 120-82-1(CAS DataBase Reference) | | NIST Chemistry Reference | Benzene, 1,2,4-trichloro-(120-82-1) | | EPA Substance Registry System | 1,2,4-Trichlorobenzene (120-82-1) |
| | 1,2,4-Trichlorobenzene Usage And Synthesis |
| Description | Trichlorobenzenes (TCBs) are synthetic chemicals that
occur in three different isomeric forms. The three chlorinated
cyclic aromatic isomers are 1,2,3-trichlorobenzene (1,2,3-TCB),
1,2,4-trichlorobenzene (1,2,4-TCB), and 1,3,5-trichlorobenzene
(1,3,5-TCB). 1,2,4-TCB is one of the 188 chemicals designated
as a hazardous air pollutant under the Clean Air Act. | | Chemical Properties | 1,2,4-Trichlorobenzene is a low-melting solid or liquid with a pleasant, aromatic odor. The Odor Threshold is 1.4 ppm. | | Physical properties | Colorless liquid with an odor similar to o-dichlorobenzene. Odor threshold concentration is 1.4 (quoted, Amoore and Hautala, 1983). Miscible with most organic solvents and oils; insoluble in water. Combustible. | | Uses | 1,2,4-Trichlorobenzene is used as a dielectric and heat transfer fluid in transformers. It acts as an intermediate, degreaser, wood preservative and solvent for dye. It is a high-temperature solvent used in gel permeation chromatography, especially for polyethylene and polypropylene. Further, it is used as a lubricant and as a synthetic transformer oil. | | Definition | ChEBI: 1,2,4-trichlorobenzene is a trichlorobenzene with chloro substituents at positions 1, 2 and 4. It is a solvent in various organic chemical reactions. | | Application | Trichlorobenzenes are primarily used as solvents in chemical manufacturing industries. 1,2,4-Trichlorobenzene is economically the most important isomer. It is used as a solvent in chemical reactions to dissolve oils, waxes, and resins. Furthermore, it is also used as a dye carrier. 1,2,4-Trichlorobenzene is a highly chlorinated aromatic solvent. It may be used as a solvent to prepare:
dimethylketene β-lactone dimer from tetramethyl-1,3-cyclobutanedione
salicyl-o-toluide by the reaction of phenyl salicylate and o-toluidine | | Synthesis Reference(s) | Journal of the American Chemical Society, 74, p. 3890, 1952 DOI: 10.1021/ja01135a052 | | General Description | Colorless liquid or white solid with a sharp chlorobenzene odor. Melting point 16.95°C (62.5°F) . | | Reactivity Profile | 1,2,4-Trichlorobenzene can react vigorously with oxidizing materials . Yields hydrogen chloride and phosgene when heated to decomposition [USCG, 1999]. | | Health Hazard | Exposures to high concentrations via inhalation are potentially hazardous to the lungs, kidneys and liver. Prolonged or repeated exposures or short exposure to high concentrations via inhalation are potentially hazardous to the lungs, kidneys and liver. Prolonged or repeated exposure to the eyes is likely to result in moderate pain and transient irritation. Prolonged or repeated contact with the skin may result in moderate irritation and possible systemic effects. Ingestion: May cause kidney and liver damage. | | Safety Profile | Poison by ingestion.
Moderately toxic by intraperitoneal route.
An experimental teratogen. Experimental
reproductive effects. Mutation data
reported. A slan irritant. Combustible when
exposed to heat or flame. Can react
vigorously with oxidizing materials. To fight
fire, use water, foam, CO2, dry chemical.
When heated to decomposition it emits
toxic fumes of Cl-. See also CHLORINATED
HYDROCARBONS,
AROMATIC. | | Potential Exposure | 1,2,4-Trichlorobenzene is used as a dye carrier, herbicide intermediate; a heat transfer medium; a dielectric fluid in transformers; a degreaser; a lubricant; as an industrial chemical; solvent, emulsifier, and as a potential insecticide against termites. The other trichlorobenzene isomers are not used in any quantity. | | Environmental fate | Biological. Under aerobic conditions, biodegradation products may include 1,2-dichlorobenzene,
1,3-dichlorobenzene, 1,4-dichlorobenzene, and carbon dioxide (Kobayashi and
Rittman, 1982). A mixed culture of soil bacteria or a Pseudomonas sp. transformed 1,2,4-trichlorobenzene
to 2,4,5- and 2,4,6-trichlorophenol (Ballschiter and Scholz, 1980). When 1,2,4-
trichlorobenzene was statically incubated in the dark at 25 °C with yeast extract and settled
domestic wastewater inoculum, significant biodegradation occurred, with gradual acclimation
followed by a deadaptive process in subsequent subcultures. At a concentration of 5 mg/L, 54, 70,
59, and 24% losses were observed after 7, 14, 21, and 28-d incubation periods, respectively. At a
concentration of 10 mg/L, only 43, 54, 14, and 0% were observed after 7, 14, 21, and 28-d
incubation periods, respectively (Tabak et al., 1981). In activated sludge, <0.1% mineralized to
carbon dioxide after 5 d (Freitag et al., 1985).
In an enrichment culture derived from a contaminated site in Bayou d’Inde, LA, 1,2,4-
trichlorobenzene underwent reductive dechlorination to 1,3- and 1,4-dichlorobenzene at relative
molar yields of 4 and 96%, respectively. The maximum dechlorination rate, based on the
recommended Michaelis-Menten model, was 4.6 nM/d (Pavlostathis and Prytula, 2000).
Surface Water. Estimated half-lives of 1,2,4-trichlorobenzene (0.5 μg/L) from an experimental
marine mesocosm during the spring (8–16 °C), summer (20–22 °C), and winter (3–7 °C) were 22,
11, and 12 d, respectively (Wakeham et al., 1983).
Photolytic. A carbon dioxide yield of 9.8% was achieved when 1,2,4-trichlorobenzene adsorbed
on silica gel was irradiated with light (λ >290 nm) for 17 h (Freitag et al., 1985).
Chemical/Physical. The hydrolysis half-life was estimated to be >900 yr (Ellington et al., 1988).
At 70.0 °C and pH values of 3.10, 7.11, and 9.77, the hydrolysis half-lives were calculated to be
18.4, 6.6, and 5.9 d, respectively (Ellington et al., 1986).
At influent concentrations of 1.0, 0.1, 0.01, and 0.001 mg/L, the GAC adsorption capacities
were 157, 77.6, 38.4, and 19.0 mg/g, respectively (Dobbs and Cohen, 1980). | | Shipping | UN2321 Trichlorobenzenes, liquid, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. | | Purification Methods | Separate it from a mixture of isomers by washing with fuming H2SO4, then water, drying with CaSO4 and slowly fractionally distilling. [Jensen et al. J Am Chem Soc 81 3303 1959, Beilstein 5 IV 664.] | | Toxicity evaluation | The liver is themain target of trichlorobenzenes irrespective of
the route of exposure. The mechanisms of liver toxicity
induced by these chemicals have not been illustrated. It might
involve intermediate arene oxides formed during initial
transformation to trichlorophenols. In addition, exposure
to 1,2,4-TCB induced porphyria in rats by inducing daminolevulinic
acid (ALA) synthetase, a rate-limiting enzyme
in the biosynthesis of heme, and also heme oxygenase, a ratelimiting
enzyme in the degradation of heme synthetase, and
therefore increasing heme production. | | Incompatibilities | Reacts violently with oxidants, acids, acid fumes; steam. | | Waste Disposal | Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced. |
| | 1,2,4-Trichlorobenzene Preparation Products And Raw materials |
| Raw materials | Sodium hydroxide-->Calcium hydroxide-->CALCIUM CARBONATE-->1,2,3-Trichlorobenzene-->1,3,5-Trichlorobenzene-->HEROIN | | Preparation Products | Anthraquinone-->Copper(II) phthalocyanine-->Tetrachlorvinphos-->Pigment Geen 7-->Naphthionic acid-->1,2,3-Trichlorobenzene-->1,3,5-Trichlorobenzene-->2,5-Dichlorobenzoic acid-->1,2-NAPHTHALIC ANHYDRIDE-->Tetradifon-->2,4,5-Trichloroaniline-->HEXACHLOROBENZENE-->1,2,4-Trichloro-5-nitrobenzene-->Trichloroacetyl isocyanate-->N-propyl-N-[2-(2,4,6-trichloro phenoxy ethyl)carbamyl chloride]-->1-BROMO-2,4-DICHLOROBENZENE-->2,2',3,4,4',5,5'-HEPTACHLOROBIPHENYL-->1-IODO-2,4,5-TRICHLOROBENZENE |
|