Preparation of ODM-201
Nov 12,2019
ODM-201 is a new generation AR inhibitor with superior preclinical efficacy compared to enzalutamide and bicalutamide, it does not enter the brain in preclinical studies, and it shows very promising activity and no significant toxicity in patients with CRPC.
ODM-201 can be prepared according to the reported literatures[1-3].
Method 1
The starting material (656.0 mg, 1.6 mmol), MeOH (8.0 ml) and THF (8.0 ml) were charged to the reaction flask. NaBH4(96.0 mg, 2.4 mmol) was added slowly, which was cooled by ice bath. The reaction was stirred for 4 h to completion followed with addition of water. The mixture was concentrated under vacuum tore move organic solvent and then DCM was added. The resulting mixture was washed with 1M HCl and 1M NaHCO3, respectively. The organic layer was dry over Na2SO4 and evaporated to dryness. After purification on silica using a solvent gradient of 50-90% ethyl acetate in hexanes, the product was obtained as a white solid (547.5 mg, yield 85.9%).
Method 2

To a 100 mL three-necked flask, 5 -(-1 -(qert -butyldimet hyl silyl)oxy)ethyl)-N-((S)- 1 -(3- (3 -chloro-4-cyanophenyl)- 1 H-pyrazol- 1 -yl)propan-2-yl)- 1 H-pyrazole-3 -carboxamide (4.0 g, 7.79 mmol) was added into THF (54 mL) at room temperature. Tetrabutylammonium fluoride (15.59 mL, 1.0 mol/L in THF, 15.59 mmol) was added dropwise at 0°C. The reaction solution was stirred for 6h. TLC showed the completion of the reaction. Then the reaction was quenched by water (60 mL) and extracted with ethyl acetate (50 mL). The organic layer was washed with water (30mL×3)and brine (30 mL×3) and concentrated under reduced pressure to obtainODM-201.Yield 2.88g (92.9percent); chemical purity: 99.6 percent. ESI-MS (m/z): 421[M+Na].‘H-NIVIR (400 MHz, DMSO-d3): =13.03(s, 1H), 8.19(d, J=8.5Hz, 1H), 8.07(s, 1H), 7.97(s, 2H), 7.81(d, J=2.3Hz, 1H), 6.93(s, 1H), 6.40(s, 1H), 5.40(d, J5.OHz, 1H),4.85-4.67(s, 1H), 4.33(m, 3H), 1.38(d, J=6.5Hz, 3H), 1.11(d, J=6.5Hz, 3H).
Method 3
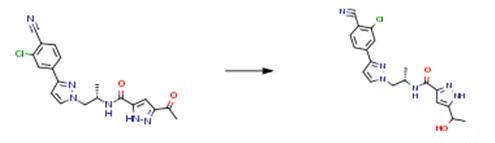
Granular sodium borohydride (15 kg) and EtOH (1370 kg) were placed into the 6.3 m3 enamel reaction vessel. The mixture was solubilized by stirring for 30 mm at 22°C. (S)-3 -acetyl-N-( 1 -(3 -(3 -chloro-4-cyano-phenyl)- 1 H-pyrazol- 1 -yl)propan-2-yl)-1H-pyrazole-5-carboxamide (225 kg) was added to the reaction vessel. The mixture was then stirred at 22 °C for 4 hours to complete the reaction. Then pH of the mixture was adjusted to acidic with HC1 in water. Water (800 kg) was then added and the pH of the mixture was set to 7.0 ± 1.0 by addition of NaOH in water.
The mixture was warmed to 65 °C and then transferred to 6.3 m3 jacketed steel reaction vessel. The mixture was warmed to 78 °C to dissolve the mixture. The solution was cooled to 64 °C under nitrogen atmosphere. The solution was seeded at 64 °C under mild stirring. The solution was then cooled during 8 h to 30 °C under mild stirring.
Thereafter water (2600 kg) was added during 7 - 10 h at 30 °C under mild stirring. The mixture was cooled during 2 h to 20 °C under mild stirring and then stirred further for 1 h. The precipitated product was isolated by centrifuge, washed with water and dried under vacuum at 40 - 60 °C to obtain 214 kg of crystalline particles with rounded particle shape.
References
1. Yu J, Zhou P, Hu M, Yang L, Yan G, Xu R, Deng Y, Li X, Chen Y. Discovery and biological evaluation of darolutamide derivatives as inhibitors and down-regulators of wild-type AR and the mutants[J]. European Journal of Medicinal Chemistry, 2019, 182:111608
2. Lianyungang Runzhong Pharamceutcial Co., Ltd., Shanghai Institute of Pharmaceutical Industry. Pan T, Xia C, Yang Y, Zhang A. Process for preparation of novel androgen receptor antagonist. WO2018/108130[P], 2018, A1. Page column 30; 31.
3. Orion Corporation. Harteva M, Staffans A. Manufacture of a crystalline pharmaceutical product. WO2018/162793[P], 2018, A1. Page column 12.
- Related articles
- Related Qustion
- The introduction of Darolutamide (ODM-201) Jan 17, 2024
Darolutamide (ODM-201) is a structurally distinct non-steroidal androgen receptor (AR) antagonist being developed by Orion and Bayer as a treatment for prostate cancer.
Brexpiprazole is an antipsychotic medication. It works by changing the actions of chemicals in the brain. It is used to treat the symptoms of schizophrenia. It is also used together with other medications to treat major depressive disorder.....
Nov 12,2019Drugs3,5-Dichlorobenzaldehyde is an important organic intermediate to synthetize substituted benzene products.....
Nov 12,2019Aromatic aldehydesODM-201
1297538-32-9You may like
- ODM-201
-

- $9.00 / 10g
- 2024-04-26
- CAS:1297538-32-9
- Min. Order: 10g
- Purity: 99%
- Supply Ability: 10 tons
- ODM-201
-

- $40.00 / 1Gram
- 2024-04-26
- CAS:1297538-32-9
- Min. Order: 1Gram
- Purity: 99.99%
- Supply Ability: 50Tons
- ODM-201
-

- $25.00 / 1kg
- 2023-08-22
- CAS:1297538-32-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100tons