Properties and Reactions of Pyrrole
Nov 14,2019
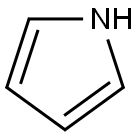
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH.It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.
Properties
Pyrrole has a nutty odor. Pyrrole is a 5-membered aromatic heterocycle, like furan and thiophene. Unlike furan and thiophene, it has a dipole in which the positive end lies on the side of the heteroatom, with a dipole moment of 1.58 D. In CDCl3, it has chemical shifts at 6.68 (H2, H5) and 6.22 (H3, H4). Pyrrole is weakly basic, with a conjugate acid pKa of −3.8. The most thermodynamically stable pyrrolium cation (C4H6N+) is formed by protonation at the 2 position.
Pyrrole is very much less basic than secondary amines but much more acidic. Pyrrole is, however, still a very weak acid (pKa = 17.5). The nitrogen-bound proton can be abstracted from pyrrole by the use of strong bases such as sodium amide in liquid ammonia and n-butyllithium in hexane. Reaction of pyrrole with Grignard reagents results in the formation of halomagnesyl derivatives 170. These nucleophiles have ambident properties and can react with electrophiles at nitrogen, C(2) or C(3). N-Unsubstituted indoles similarly form metal derivatives when treated with a variety of reagents such as n-butyllithium, sodamide, Grignard reagents and thallium(I) ethoxide
Reactions
Pyrrole, being a π-rich heterocycle, is not easily reduced catalytically. However, catalytic reduction is accomplished at moderate temperature and pressure using Pd, Pt, or Raney Ni catalysts. The presence of an electron-withdrawing N-substituent facilitates reduction of the pyrrole ring, while in the case of C-substituted pyrrole, preferential autolytic reduction of substituent takes place.
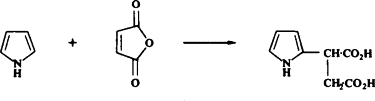
Catalysts such as copper chromite are capable of reducing the pyrrole ring as well as substituents present on the ring. However, catalysts such as rhodium and aluminum only reduce the pyrrole ring leaving substituents unaffected.
Chemical reduction of pyrrole with Zn/HCl gave a mixture of ∆1-, ∆2-, and ∆3-pyrrolines in which the latter was the major product. ∆1- and ∆2-Pyrrolines further reduced to pyrrolidine. Reduction of pyrrole with hydriodic acid and phosphorus gave pyrrolidine. However, when Na–ethanol was used as reducing agent, substituents were reduced preferentially compared to the pyrrole ring. Sodium-ethanol reduction of 1-arylpyrrole afforded 1-(1-pyrrollyl)cyclohexa-1,4-diene by reducing the aryl ring leaving the pyrrole ring unaffected.
- Related articles
- Related Qustion
- Application of Pyrrole Jan 21, 2022
Pyrrole is isoelectronic with a cyclopentadienyl anion but is electrically neutral because of the higher nuclear charge on nitrogen. The heteroatoms, N, O, and S, in the ring can be either neutral or carry a positive charge. In fact, hetero
Potassium carbonate, K2CO3, appears as a white powder or as colorless solid crystal and has a salty taste. Also known as potash or pearl ash, it may be used in pharmaceutical laboratories as a drying agent or as a source of potassium.....
Nov 14,2019APIThe formation of acetoin (acetylmethylcarbinol) by various tissues has been shown to be a synthetic process, the 4-carbon ketol originating from pyruvic acid in bacteria (1, 2), from pyruvic acid and acetaldehyde in yeast (3) and animal tis....
Nov 14,2019Organic ChemistryPyrrole
109-97-7You may like
- Pyrrole
-

- $5.00 / 1KG
- 2025-05-26
- CAS:109-97-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10000kg
- Pyrrole
-

- $1.50 / 1g
- 2023-07-27
- CAS:109-97-7
- Min. Order: 1g
- Purity: 99.0% Min
- Supply Ability: 100 Tons
- Pyrrole
-
- $30.00/ Kg
- 2023-05-31
- CAS:109-97-7
- Min. Order: 1Kg
- Purity: 99.0% up
- Supply Ability: 50 tons per month