ピロール 化学特性,用途語,生産方法
外観
無色~褐色, 澄明の液体
性質
ピロールの融点は-24℃、沸点は129.79℃です。水に溶けにくく、有機溶媒に溶解します。濃塩酸などと反応して重合します。
ピロールの窒素原子の塩基性は、ピリジンやアミンと比較するととても低いです。理由として、窒素原子が有する孤立電子対が、環全体に非局在化していることが挙げられます。
溶解性
水に微溶 (8g/100g水, 25℃)。有機溶媒に可溶。エタノール及びジエチルエーテルに溶け、水にほとんど溶けない。
解説
ピロール,アゾールともいう.コールタール中に少量,骨油中にかなり多量に存在する.2-フランカルボン酸アンモニウムの加熱,フランとアンモニアをAl2O3上で加熱,あるいはスクシンイミドを亜鉛末と蒸留するなどの方法で生成する.クロロホルム様の特有な臭気をもつ無色の液体.沸点130~131 ℃.d204 0.9691.n20D 1.5085.λmax 209,240 nm(ε 6730,300).有機溶媒に易溶,水に難溶.空気中で着色する.そのNH基の塩基性はきわめて弱い(pKb 13.6)が,反応性は高く,酸性では重合が起こって樹脂化しやすい.金属化合物はピロール誘導体の合成に用いられる.たとえば,ピロール環は芳香族性を有し,環の水素のハロゲン置換,ジアゾニウム塩のカップリング,酸無水物によるアシル化などが容易に進行する.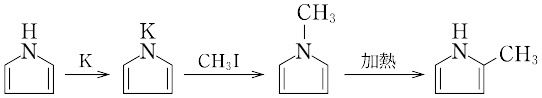
ピロールという名称はギリシア語の“火のように赤い”という意味であり,ピロールおよびその誘導体は希塩酸溶液中でピロール赤を生成する.亜セレン酸による酸化反応やケイ酸,モリブデン酸アンモニウムおよび硫酸の混合溶液との反応では濃青色のピロール青を呈するので,これらの検出にも用いられる.
用途
有機合成、腐食防止剤、ポリマーの製造、電解質、溶剤。
用途
亜セレン酸、ケイ酸の検出試薬。亜セレン酸により酸化されてピロール青の濃青色を呈する。リン酸、鉄(3)を共存させ、これを酸化触媒として用いた場合、限界濃度0.1ppm。ケイ酸、モリブデン酸アンモニウム、硫酸の混合溶液は試薬と反応して青色を呈する。
構造
ピロールの分子式はC4H5Nで、分子量は67.09、密度は0.967g/cm3です。分子内の部分構造としてピロールを含む化合物は非常に多いです。ピロールの部分構造は、ピロール環と呼ばれています。
合成
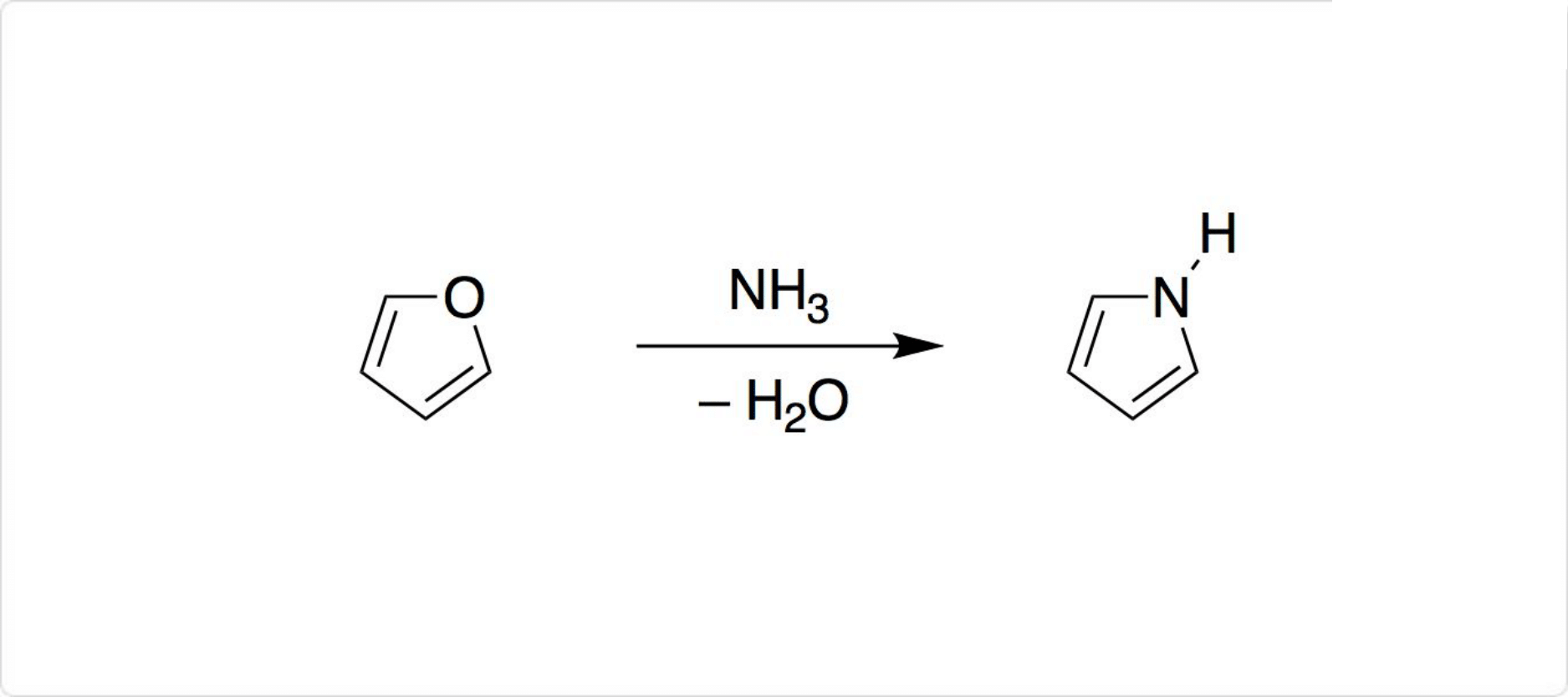
図2. ピロールの合成
ピロールは、を触媒として、フランとを反応させると生成可能です。ピロリジンの接触脱水素によっても合成できます。
それ以外にも、ピロール環の合成法が多数知られています。例えば、ハンチュのピロール合成では、β-ケトエステル、α-ハロケトン、アンモニアを用いて、置換ピロールが生成可能です。
また、クノールのピロール合成によって、カルボニル基のα位にメチレン基を持つ化合物とα-アミノケトンから、置換ピロールが得られます。さらに、パール・クノール合成では、1,4-ジカルボニル化合物からフランを経由して、ピロールが生じます。
使用上の注意
不活性ガス封入
化学的特性
Six π-electrons are distributed over the five ring atoms of pyrrole. Delocalization
of these electrons stabilizes the ring and the lone pair of electrons on the nitrogen
atom, which is responsible for the usual basicity of nitrogen compounds, is
involved in the electron cloud, and is not available for sharing. Hence, pyrrole is an extremely weak base and the pyrrolic nitrogen is not readily susceptible to
electrophilic enzymic attack (Damani, 1985). There is a high electron density,
however, at all positions of the ring, which causes pyrrole to be reactive toward
electrophilic substitution. In general, electrophilic substitution reactions on the
neutral molecule occur preferentially at the C-2 or C-5 positions (Jones and Bean,
1977; Damani and Crooks, 1982).
物理的性質
Pyrrole is a colorless to brown liquid that has a sweet, warm-ethereal smell, similar to chloroform. It dissolves in ethanol, ether, benzene, dilute acids, and most non-volatile oils but does not dissolve in water or dilute alkalis. When stored for extended periods, it tends to aggregate and become brown due to the influence of light.
定義
ChEBI: 1H-pyrrole is a tautomer of pyrrole that has the double bonds at positions 2 and 4. It is a pyrrole and a secondary amine. It is a tautomer of a 2H-pyrrole and a 3H-pyrrole.
調製方法
Pyrrole originally was prepared industrially by fractional distillation of coal tar,
bone oil or other protein material, and purified through formation of its potassium
derivative (Runge, 1834; Michelman, 1925). Later it was produced by heating
ammonium mucate with glycerol or mineral oil (Blicke and Powers, 1927;
McElvain and Bollinger, 1941). It is now manufactured by addition of ammonia to
either acetylene or butadiene. Good yields of pyrrole also may be obtained from
the reaction of ammonia with the corresponding heterocyclic compound (furan) in
a vapor-phase process at 480° to 500°C, using alumina as a catalyst (Thompson,
1972) or by catalytic reaction of furan with ammonia over a molybdenum or
vanadium oxide catalyst at 350-400°C (Bishop and Denton, 1950).
製造方法
By fractional distillation of bone oil (bone oil is obtained by destructive distillation of animal bone) and subsequent purification via the corresponding potassium salt; by thermal decomposition of ammonium mucate in glycerol or mineral oil.
一般的な説明
Pyrrole is one of the flavor compounds that is formed in thermally processed foods due to the Maillard reaction.
危険性
Moderate fire risk. Toxic by ingestion and
inhalation.
健康ハザード
Pyrrole is harmful if swallowed, inhaled, or absorbed through the skin. Its vapor or
mist is irritating to the eyes, mucous membranes and upper respiratory tract
(Lenga, 1985; Sax, 1984). Although no cases of occupational disease due to
pyrrole have been reported, it has a depressant action on the central nervous
system and, in severe intoxication, it is injurious to the liver. Tests indicate that it
has moderate cumulative toxicity (Parmegianni, 1983).
火災危険
Combustible liquid; flash point (closed cup)
39°C (102°F); vapor forms explosive mixtures
with air; LEL and UEL values are not
available. Heating with strong oxidizers can
be violent.
化学性质
黄赤色透明液体,融点?23℃,沸点131℃
使用用途
ピロールは、有機合成やポリマーの製造、鉄鋼材料の腐食防止剤、などの電解質、および溶剤として利用されています。
また、ピロールとアルデヒドを酸性条件で縮合するとポルフィリンが合成可能です。ポルフィリンは、導電性や発光性などの特徴を持っています。
この性質により、実験でとして用いられるとともに、太陽電池や有機ELの発光材料への応用などが検討されています。そのほか、ピロールは、亜セレン酸やケイ酸の検出試薬としても使用可能です。
関連する反応
ピロールの反応
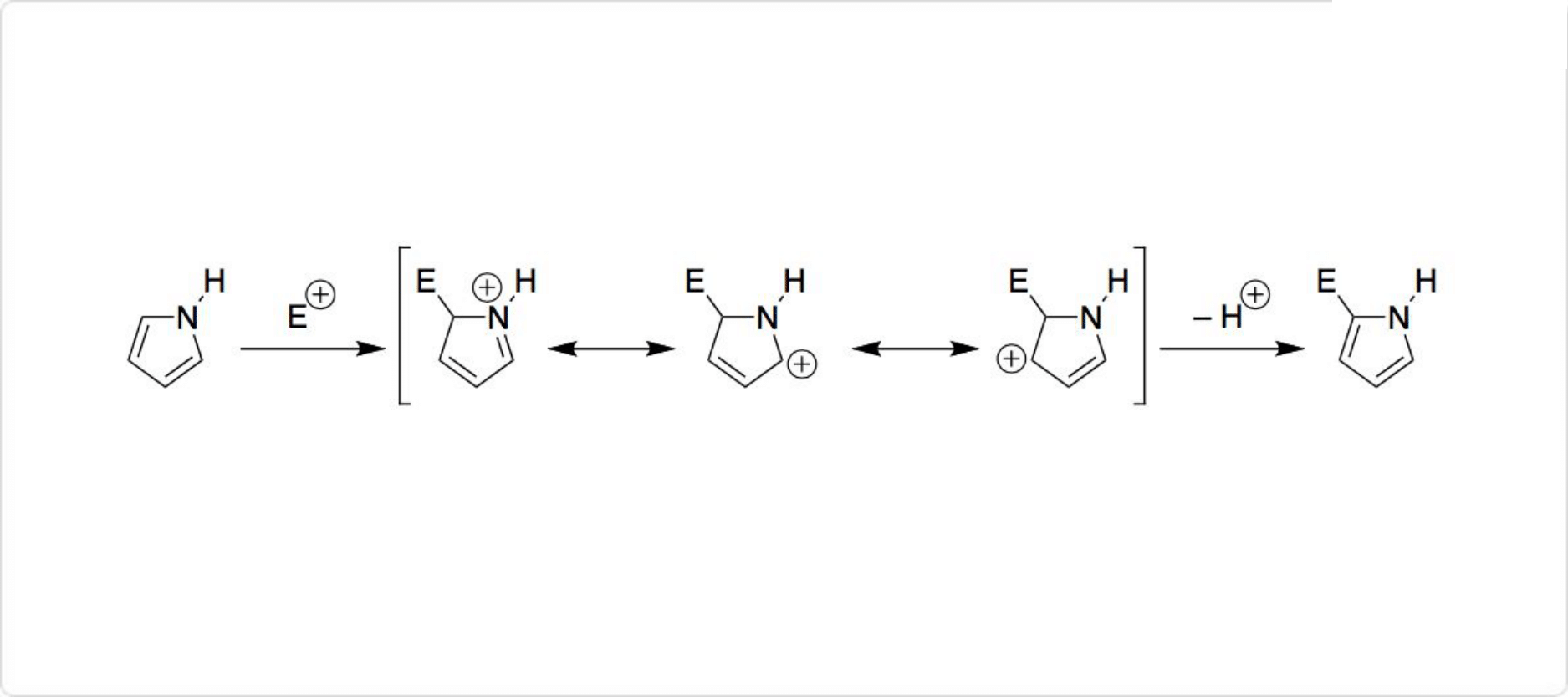
図3. ピロールの反応
ピロールは芳香族であり、反応性はベンゼンやアニリンに似ています。一般的なオレフィンのような水素化が起こりにくく、通常ジエンとしてのディールス・アルダー反応も進行しません。
その一方で、アルキル化やアシル化は起こりやすいです。それに加えて、酸性条件下でピロールは、容易に重合します。
ピロールは、プロトン化された中間体の安定性が高いα位で、求電子剤と反応します。具体的には、ニトロ化剤 (HNO3/Ac2Oなど) 、スルホン化剤 (Py・SO3) 、ハロゲン化剤 (Br2、SO2Cl2、KI/H2O2など) と反応しやすいです。
酸性
1. ピロールの酸性
ピロールの窒素原子に結合している水素原子は、pKaが16.5であり、やや酸性を示します。そのため、ブチルリチウムや水素化ナトリウムなどの強塩基を用いて、脱プロトン化が可能です。生成したアニオンは求核性を有し、ヨードメタンなどの求電子試薬と反応すると、N-メチルピロールが得られます。
脱プロトン化したピロールは、配位金属の種類によって、窒素原子上または炭素原子上で求電子試薬と反応可能です。リチウム、ナトリウム、カリウムなどの金属では、N-アルキル化が進行します。それに対して、MgXなどの場合には、C-アルキル化が起こります。
工業用途
Pyrrole is used to a limited extent as a solvent for polymeric esters, but its primary
value lies in its function as a chemical intermediate. It is used in the synthesis of
non-heterocyclic compounds (Kozikowski, 1984) and its derivatives have been
used in the manufacture of dyes, herbicides, perfumes, and as cross-linking agents
for curing resins (Thompson, 1972). Derivatives of pyrrole are utilized in pharmaceutical
applications, particularly as anti-inflammation drugs and drugs with
central nervous system activity, including antihypertensive effects (Sundberg,
1984); and as antimicrobial agents (Freeman, 1975), such as fungicides (Zirngibl,
1983) and bactericides (Bailey and Johnson, 1973; Bailey et al 1973; Sundberg,
1984). Polymers of pyrrole have been used in the preparation of photoconductive
materials. The main utility of poly(pyrrole) has been for the modification of
electrode surfaces, although numerous other applications can be envisioned (Heilmann
and Rasmussen, 1984).
安全性プロファイル
Poison by ingestion, subcutaneous, and intraperitoneal routes. Flammable liquid when exposed to heat or flame; can react with oxilzing materials. To fight fire, use foam, CO2, dry chemical. Violent reaction with 2-nitrobenzaldehyde.
When heated to decomposition it emits highly toxic fumes of NOx.
純化方法
Dry pyrrole with NaOH, CaH2 or CaSO4. Fractionally distil it under reduced pressure from CaH2. Store it under nitrogen as it turns brown in air. Redistil it immediately before use. The picrate forms orange-red crystals with m 69o(dec). [Beilstein 20 H 4, 20 I 3, 20 II 3, 20 III/IV 61, 20/5 V 3.]
ピロール 上流と下流の製品情報
原材料
準備製品