Tetrabutylammonium Bromide-Activity & Application
Nov 25,2019
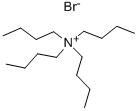
Tetrabutylammonium bromide (TBAB), a commonly used phase transfer catalyst (PTC), is a quaternary ammonium salt with a bromide counterion. Shaped as white crystals or powders, TBAB is deliquescent and have a special odor, soluble in water, alcohol and acetone. widely used in organic synthesis, TBAB act as a catalyst that transfers a reaction of a water phase (or organic phase) to an organic phase (or water phase), a catalyst that enables the reaction between the water phase and the organic phase. Possessing the properties of changing the degree of solventization of ions, TBAB could increase the activity of ion reaction, and speed up the reaction speed. In the past, there is a problem that reactants in two phases are difficult to react. Now, with the presence of PTC, the rampart could be removed. Acting as the cationic surfactant of choice, TBAB were chosen in drug formulations to reduce drug-drug interactions and to enhance the bioavailability of poorly water-soluble lifesaving drugs. A study on the inclusion behaviour of TBAB with β-CD host would therefore, have high scientific value for the development of novel TBAB based pharmaceutical formulations. The thermal stability of TBAB was found to increase after complexation.
Effectively used in organic synthesis, TBAB plays an irreplaceable role. For example, in the preparation of R2C-type compounds, which could further used in the production of corresponding nitrile, isonitrile, halothane, dichlorocyclopropane derivatives, hydroxyl carboxylic acid and diazomethane, etc., TBAB act as the reaction auxiliary. In alkylation reaction, compared with the traditional method, it can avoid the harsh conditions such as anhydrous operation, and the product yield is higher. It can also be used in REDOX reaction, ester hydrolysis, cyanohalogen ion replacement reaction, condensation reaction, addition reaction, polymerization reaction, carbene ring addition reaction and elimination reaction.
In the other hand, TBAB is an ion pair reagent for the synthesis of bamicillin, sutaicillin, etc. It colud also be used as a polar-spectrum analysis reagent and to prepare many other tetrabutylammonium salts via salt metathesis reactions. By complexing tetrabutylammonium bromide and polyethylene glycol, deep eutectic solvents were prepared.
References
[1] Luo, X.; et al. Nitrogenous Compounds Produced by the Deep-Sea Derived Fungus Leptosphaeria sp. SCSIO 41005. Natural Product Communications, 2018, 13 (6): 677-688.
[2] Li, K.; et al. New nitrogen-containing bromophenols from the marine red alga Rhodomela confervoides and their radical scavenging activity. Food Chem., 2012, 135, 868–872.
[3] Ahmad, H.; et al. Selective dual cholinesterase inhibitors from Aconitum leave. Journal of Asian Natural Products Research, 2018, 20(2), 172-181.
[4] Guo, J.; et al. Synthesis of Methyl(3S)-3-(tert-Butoxycarbonyl amino)-4-oxobutanoate. Chinese Journal of Pharmaceuticals, 2012, 43(3): 172-174.
[5] Liu, C.-R.; et al. Concise asymmetric synthesis of (-)-deoxoprosophylline. Tetrahedron Asymmetry, 2008, 19(23), 2731-2734.
- Related articles
- Related Qustion
- Tetrabutylammonium Bromide: A Versatile Phase-Transfer Catalyst for Efficient Synthesis of Bioactive N-Heterocycles Jul 3, 2024
Tetrabutylammonium bromide, a versatile catalyst, enhances efficiency in synthesizing bioactive compounds like N-heterocycles, showcasing broad utility in modern synthetic chemistry.
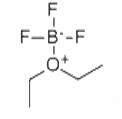
Boron trifluoride etherate, strictly boron trifluoride diethyl etherate, or boron trifluoride–ether complex, is the chemical compound with the formula BF3O(C2H5)2, often abbreviated BF3OEt2.....
Nov 25,2019Organic reagentsCopper sulfate, also known as copper sulphate is an inorganic compound with the chemical formula CuSO4. Other names for this chemical compound include Roman vitriol, bluestone, blue vitriol and vitriol of copper. Hydrated copper sulfate is....
Nov 25,2019Inorganic chemistryTetrabutylammonium bromide
1643-19-2You may like
Tetrabutylammonium bromide manufacturers
- Tetrabutylammonium bromide
-

- $1.00 / 1g
- 2025-12-20
- CAS:1643-19-2
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 100kg
- Tetrabutylammonium bromide
-
- $37.00 / 1kg
- 2025-12-19
- CAS:1643-19-2
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 100kg
- Tetrabutylammonium bromide
-

- $0.00 / 1KG
- 2025-12-19
- CAS:1643-19-2
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 30tons/month