Toxicity hazards of Dichloromethane
Nov 18,2021
Dichloromethane is also known as methine chloride, methylene chloride and chloromethylene. The molecular formula is CH2Cl2, a colorless and volatile liquid with an aromatic smell similar to ether, which is irritating. Soluble in ethanol and ether, slightly soluble in water.
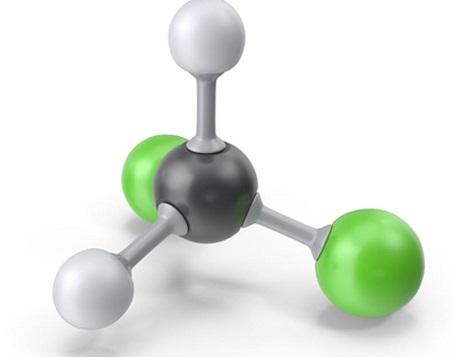
Dichloromethane can be used as a solvent, dental local anesthetic, refrigerant and fire extinguishing agent. In addition to being used in organic synthesis, dichloromethane is often used as a non-flammable solvent for flammable materials such as cellulose acetate and motion picture film. It can also be used as a paint remover, extractant, fire extinguishing agent, refrigerant, etc.
Toxic effects
1. General toxicity
The oral median lethal dose of DCM in mice is 2640 mg/kg, the median lethal concentration of DCM in mice and rats are 51860 and 74760 mg/m3, respectively, and the rat accumulation coefficient is greater than 5. DCM has a irritating effect on human skin and can burn the skin in severe cases; DCM has an irritating effect on rabbit eyes, which can cause eyes to shed tears for a long time while increasing intraocular pressure, causing inflammatory reactions such as conjunctival hyperemia, edema and increased secretions, and the dose is healed The degree of major damage is also more serious; at the same time, it will also lead to an increase in the thickness of the cornea.
2. Genotoxicity
In the air exposure experiment of Ames, both TA98 and TA100 strains showed mutagenic positive results in the DCM environment with a mass concentration of 22.17~709.33 mg/L, indicating that DCM is mutagenic and can cause gene mutations.
3. Carcinogenicity
Epidemiological studies have found that the carcinogenicity of DCM is mainly concentrated in the liver and lungs, and liver cancer has become the focus of research on its toxic effects. Studies have shown that the potential carcinogenicity of DCM may be related to the process of GST-T1 being metabolized into formaldehyde, and DCM can cause an increase in the incidence of human non-Hodgkin’s lymphoma (OR=4.42, 95%CI 2.03-9.62). Other studies have shown that DCM makes the intermediate metabolites of GST interact with DNA, affecting cell mitosis, and causing the spread of lung cancer cells in mice. However, the exact carcinogenic mechanism of DCM is currently unclear.
4. Reproductive and developmental toxicity
Mice were given intravenous inhalation of DCM with mass concentrations of 0, 2593, 5186 and 10372 mg/m3 daily for 2 hours for 6 consecutive days. The study showed that there was no statistically significant difference in the birth weight of infants born to workers in the DCM exposure group and the control group (P>0.05). The above research results show that in a certain concentration range, DCM has certain reproductive toxicity, but no obvious developmental toxicity has been found.
5. Neurotoxicity
The Kunming mice were divided into 34 dose groups of 0, 1897, 12806 and 32015 mg/m. The mice were exposed to the poison by conventional static inhalation for 7 days. Other studies have shown that exposure of workers to DCM can decrease part of their neurobehavioral functions, inhibit central nervous system function, interfere with central nervous system excitation-inhibition balance, and reduce serum acetylcholinesterase levels.
6. Immune toxicity
DCM has an inhibitory effect on the immune function of animals and humans. Studies have shown that the thymus and spleen of mice in the DCM-exposed group were atrophy, and the quality of their thymus and spleen was lower than that of the control group (P<0.05); the positive rate of acid α-naphthyl acetate in peripheral blood lymphocytes of the mice in the DCM-exposed group Compared with the control group (P<0.05). Another study showed that the levels of immunoglobulin G in workers exposed to DCM were higher than those in the control group (P<0.01); the lymphocyte turnover rate and immunoglobulin M levels were lower than those in the control group (P<0.01).
7. Other toxicity
The mechanism of DCM's toxicity to the heart may be that DCM is metabolized into carbon monoxide in the body, which reduces the blood's ability to transport oxygen to the heart muscle, thereby causing a certain toxicity to the heart; it may also be that the toxicity of DCM directly affects the heart. DCM can stimulate the lungs, and in severe cases can cause pulmonary edema. Patients with DCM poisoning often experience respiratory irritation such as chest tightness, shortness of breath, cough, and expectoration. On auscultation of the lungs, rales and breath sounds weakened. A computerized tomography scan of the lungs showed thickening of the pleura, increased and blurred lung texture, and effusion and exudative changes in the lungs.
- Related articles
- Related Qustion
- Is CH2Cl2 Polar or Nonpolar? Dec 20, 2023
An electronegativity difference of 0.61 units exists between a carbon and a chlorine atom in the C-Cl bond,makes dichloromethane a polar molecule.
- Toxicity of Dichloromethane Jan 18, 2022
Dichloromethane (DCM; mol. wt. 93.328) was first prepared in 1840 by mixing chloromethane and chlorine and exposed to sunshine. It has been used as a versatile solvent to dissolve various organic compounds in many chemical processes since W
- Uses of Dichloromethane Nov 20, 2019
Dichloromethane is a geminal organic chemical. It is also known to the scientists under the names methylene chloride or methylene dichloride. The substance may also be called refrigerant-30 freon-30, R-30, DCM, UN 1593, solmethine, narkotil
A procarcinogen, benz[a]anthracene is metabolized to its carcinogenic form by phase 1 and phase 2 metabolism. As with other polycyclic aromatic hydrocarbons (PAHs), the presence of the ‘bay’ region contributes greatly to the carcinogenicity....
Nov 18,2021Organic reagentsBenzene is a clear, colorless, highly flammable and volatile, liquid aromatic hydrocarbon with a gasoline-like odor. Benzene is found in crude oils and as a by-product of oil-refining processes. In industry benzene is used as a solvent, as....
Nov 19,2021Organic ChemistryDichloromethane
75-09-2You may like
- DCM
-

- $0.00 / 25KG
- 2025-05-16
- CAS:75-09-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- Dichloromethane
-
- $0.00 / 200kg
- 2025-05-14
- CAS:75-09-2
- Min. Order: 20kg
- Purity: 99.0%
- Supply Ability: 20 tons
- Dichloromethane
-

- $450.00 / 10ton
- 2025-05-02
- CAS:75-09-2
- Min. Order: 50ton
- Purity: 99
- Supply Ability: 500 ton