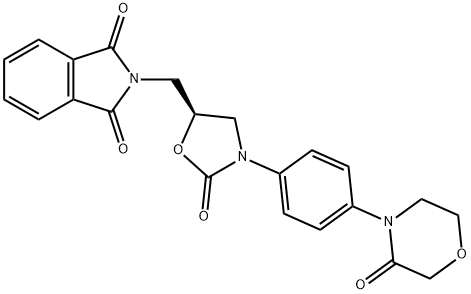
What is 1H-isoindole-1,3(2H)-dione, 2-[[(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-5-oxazolidinyl]methyl]- used for?
Feb 27,2020
Rivaroxaban Intermediate
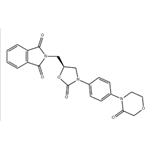
1H-Isoindole-1,3(2H)-dione, 2-[[(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-5-oxazolidinyl]methyl]- (C22H19N3O6, CAS registry No. 446292-08-6) is also called 2-({(5S)-2-Oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)-1H-isoindole-1,3(2H)-dione, which is a white solid. Its flash point is 378.92 oC.
Fig 1. Chemical structure formula of 1H-isoindole-1,3(2H)-dione, 2-[[(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-5-oxazolidinyl]methyl]-
It is an important intermediate for the synthesis of rivaroxaban, which is an anticoagulant and the first orally active direct factor Xa inhibitor[1]. The drug was developed and manufactured by Bayer and was marketed in the USA by Janssen Pharmaceuticals, a unit of Johnson & Johnson. It is used for prophylaxis and treatment of thromboembolic disease. Rivaroxaban is an oral anticoagulant, which is orally administered as tablets containing 10 mg, 15 mg and 20 mg. Rivaroxaban is safe and highly effective compared with heparin anticoagulant drugs. Sales of rivaroxaban have increased rapidly year after year, and global sales reached 654 million US dollars in 2017[2].
Synthesis
It can be synthesized from 4-(4-aminophenyl)morpholin-3-one and bis(trichloromethyl)carbonate (triphosgene) to give 4-(4-isocyanatophenyl)morpholin-3-one, followed by cyclization with (S)-2-(oxiran-2-ylmethyl)isoindoline-1,3-dione. Then, hydrazinolysis of 1H-isoindole-1,3(2H)-dione, 2-[[(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-5-oxazolidinyl]methyl]- in the presence of hydrazine hydrate yields (S)-4-(4-(5-(aminomethyl)-2-oxooxazolidin-3-yl)phenyl)morpho-lin-3-one, which undergoes N-acylation with 5-chlorothiophene-2-carbonyl chloride to give rivaroxaban[3].
Process-related Impurities
To ensure product quality and minimize safety risks, process-related impurities should be observed throughout the development lifecycle of rivaroxaban as the chemistry and process understanding evolve. It has been reported that seven process-related impurities were identified and synthesized. These structures are confirmed by nuclear magnetic resonance spectroscopy and high-resolution mass spectrometry (HRMS). All impurities could be effectively removed by recrystallization in a mixture of DMSO and water. During the deprotection reaction in the synthetic process of rivaroxaban, intermediate 1H-isoindole-1,3(2H)-dione, 2-[[(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-5-oxazolidinyl]methyl]- was treated with excess hydrazine hydrate at 90°C to give the amino intermediate. The impurities (S)-2-(2-((4-(5-(aminomethyl)-2-oxooxazolidin-3-yl)phenyl)amino)ethoxy)acetohydrazide and (S)-4-(4-(((2-oxooxazolidin-5-yl)methyl)amino)phenyl)morpholin-3-one could be generated in this step, which are confirmed and distinguished from their isomers by single-crystal diffraction[3b]. Due to the poor stability of the amino intermediate, impurity (S)-4-(4-(((2-oxooxazolidin-5-yl)methyl)amino)phenyl)morpholin-3-one was the degradation product of the amino intermediate under high-temperature conditions, which may cause the production of another impurity in the subsequent reaction.
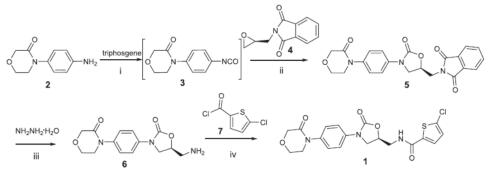
Synthesis of rivaroxaban with 1H-isoindole-1,3(2H)-dione, 2-[[(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-5-oxazolidinyl]methyl]- as the intermediate.
References
[1]. (a) Gulseth, M. P.; Michaud, J.; Nutescu, E. A., Rivaroxaban: an oral direct inhibitor of factor Xa. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists 2008, 65 (16), 1520-9; (b) Roehrig, S.; Straub, A.; Pohlmann, J.; Lampe, T.; Pernerstorfer, J.; Schlemmer, K. H.; Reinemer, P.; Perzborn, E., Discovery of the novel antithrombotic agent 5-chloro-N-({(5S)-2-oxo-3- [4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene- 2-carboxamide (BAY 59-7939): an oral, direct factor Xa inhibitor. Journal of medicinal chemistry 2005, 48 (19), 5900-8.
[2]. Global rivaroxaban market growth at a cagr of 8.2% during 2018-2025. https://express-press-release.net/news/2018/06/19/275877.
[3]. (a) Tian, S.; Tang, B.; Zhang, M.; Gao, Q.; Chen, B.; Zhang, Q.; Xu, G., An Improved Synthesis of Rivaroxaban. Organic Preparations and Procedures International 2017, 49 (2), 169-177; (b) Yu, J.; Qiu, P. C.; Ke, B.; Chen, H.; Zhao, C. M.; Zhang, F. L., Identification and Synthesis of Impurities During a Novel Process Development of Rivaroxaban. Journal of Heterocyclic Chemistry 2018, 55 (12), 2852-2858.
- Related articles
- Related Qustion
- What is 1H-isoindole-1,3(2H)-dione, 2-[[(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-5-oxazolidinyl]methyl]-? Aug 11, 2020
1H-isoindole-1,3(2H)-dione, 2-[[(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-5-oxazolidinyl]methyl]- is an important intermediate for the synthesis of rivaroxaban, which is an anticoagulant and the first orally active direct factor Xa inhib
Methyl benzoylformate, the methyl ester of phenylglyoxylic acid with methanol, can be reduced with many methods.....
Feb 27,2020Organic reagents1,3,5-triglycidyl isocyanurate (TGIC) is a white powder or granular solid and is normally sold under the trade name ARALDITE PT810 or TEPIC G.....
Feb 28,2020Organic reagentsYou may like
- (R)-2-((2-oxo-3-(4-(3-oxomorpholino)phenyl)oxazolidin-5-yl)methyl)isoindoline-1,3-dione
-
- $0.00 / 1kg
- 2025-04-04
- CAS:446292-08-6
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1Ton
- 2-[[(5S)-2-Oxo-3-[4-(3-oxo-4- morpholinyl)phenyl]-5- oxazolidinyl]methyl]-1H-isoindole- 1,3(2H)-dione
-
![446292-08-6 2-[[(5S)-2-Oxo-3-[4-(3-oxo-4- morpholinyl)phenyl]-5- oxazolidinyl]methyl]-1H-isoindole- 1,3(2H)-dione](/ProductImageEN/2023-05/Small/05f49211-a3ea-4865-ad45-51bd023f13c6.png)
- $0.00 / 1Kg
- 2023-05-26
- CAS:446292-08-6
- Min. Order: 1Kg
- Purity: 99%
- Supply Ability: 1-10000kgs
- 1H-ISOindole-1,3(2H) -Dione, 2-[[(5S)-2-OXO-3-[4-(3-OXO-4-MORPHolinyl)PHENYL] -5-OXAZolidinyl]METHYL]-
-
![446292-08-6 1H-ISOindole-1,3(2H) -Dione, 2-[[(5S)-2-OXO-3-[4-(3-OXO-4-MORPHolinyl)PHENYL] -5-OXAZolidinyl]METHYL]-](/ProductImageEN/2022-10/Small/c7b2a50b-2869-4b05-9579-4b4274bc9fa5.png)
- $30.00/ Kg
- 2022-10-07
- CAS:446292-08-6
- Min. Order: 1Kg
- Purity: 99.0% up
- Supply Ability: 50 tons per month