| Identification | Back Directory | [Name]
2-(Dicyclohexylphosphino)-3,6-dimethoxy-2'-4'-6'-tri-i-propyl-1,1'-biphenyl, min. 98% BrettPhos | [CAS]
1070663-78-3 | [Synonyms]
BrettPhos 96%
2-(Dicyclohexylphosphino)-3,6-diMethoxy-2'-4'-6'-tri-i-propyl-1,1'
2-Dicyclohexylphosphino-2',4',6'-triisopropyl-3,6-dimethoxybiphenyl
2-Dicyclohexylphosphino-2',4',6'-triisopropyl-3,6-diMethoxybiphenyl, 97+%
2-(Dicyclohexylphosphino)3,6-dimethoxy-2′,4′,6′-triisopropyl-1,1′-biphenyl
2-(Dicyclohexylphosphino)-3,6-dimethoxy-2'-4'-6'-tri-i-propyl-1,1'-bipheny
2-(Dicyclohexyl phosphino)-3,6-dimethoxy-2',4',6'-tri-i-propyl-1,1'-biphenly
dicyclohexyl-[3,6-dimethoxy-2-[2,4,6-tri(propan-2-yl)phenyl]phenyl]phosphane
2-(Dicyclohexylphosphino)-3,6-diMethoxy-2'-4' -6'-tri-i-propyl-1,1'-biphenyl
Dicyclohexyl(2',4',6'-triisopropyl-3,6-diMethoxy-[1,1'-biphenyl]-2-yl)phosphine
dicyclohexyl[3,6-dimethoxy-2',4',6'-tris(propan-2-yl)-[1,1'-biphenyl]-2-yl]phosphane
Dicyclohexyl[3,6-dimethoxy-2',4',6'-tris(1-methylethyl)[1,1'-biphenyl]-2-yl]phosphine
2-(Dicyclohexylphosphino)-3,6-dimethoxy-2'-4'-6'-tri-i-propyl-1,1'-biphenyl(BrettPhos)
2-Dicyclohexylphosphino-2',4',6'-tri-i-propyl-3,6-dimethoxy-1,1'-biphenyl, (BrettPhos)
2-Dicyclohexylphosphino-2',4',6'-tri-i-propyl-3,6-diMethoxy-1,1'-biphenyl, 98% (BrettPhos)
2-(Dicyclohexylphosphino)-3,6-dimethoxy-2'-4'-6'-tri-i-propyl-1,1'-biphenyl, min. 98% BrettPhos | [Molecular Formula]
C35H53O2P | [MDL Number]
MFCD11973797 | [MOL File]
1070663-78-3.mol | [Molecular Weight]
536.768 |
| Chemical Properties | Back Directory | [Melting point ]
191-193℃ | [Boiling point ]
607.7±55.0 °C(Predicted) | [storage temp. ]
Refrigerator, under inert atmosphere | [solubility ]
Chloroform (Slightly), Methanol (Slightly, Sonicated) | [form ]
crystal | [color ]
white | [InChI]
1S/C35H53O2P/c1-23(2)26-21-29(24(3)4)33(30(22-26)25(5)6)34-31(36-7)19-20-32(37-8)35(34)38(27-15-11-9-12-16-27)28-17-13-10-14-18-28/h19-25,27-28H,9-18H2,1-8H3 | [InChIKey]
WDVGNXKCFBOKDF-UHFFFAOYSA-N | [SMILES]
P(C1CCCCC1)(C1CCCCC1)C1=C(OC)C=CC(OC)=C1C1=C(C(C)C)C=C(C(C)C)C=C1C(C)C |
| Hazard Information | Back Directory | [Uses]
Ligand for the Suziki-Miyaura coupling of tosylates and mesylates. | [reaction suitability]
reagent type: catalyst
reaction type: Cross Couplings | [Synthesis]
Example 2: General procedure for the synthesis of compounds 1 to 5; A 25 mL round bottom flask was dried in an oven equipped with a magnetic stir bar and 2-iodo-2',4',6'-triisopropyl-3,6-dimethoxy-1,1'-biphenyl (1 g, 2.15 mmol) was added. The flask was evacuated and backfilled with argon and the process was repeated three times. Anhydrous THF (10 mL) was added via syringe and the reaction mixture was cooled to -78 °C. Over 10 min, n-butyllithium (2.5 M hexane solution, 940 μL, 2.36 mmol) was added dropwise. After stirring the reaction mixture for 30 min, dicyclohexylphosphonium chloride (2.26 mmol) was added dropwise over 10 min. Stirring was continued at -78 °C for 1 h. The mixture was then slowly warmed to room temperature and stirred for an additional 1.5 h. The reaction mixture was then stirred for 1.5 h. The reaction mixture was then heated to room temperature. The reaction mixture was filtered through a diatomaceous earth plug, which was pre-placed on silica (eluting with ethyl acetate). The filtrate was concentrated on a rotary evaporator to give a white solid. The crude product was recrystallized by acetone (ligands 2 to 5 were recrystallized by methanol) to give white crystals of the target product 2-(dicyclohexylphosphino)-3,6-dimethoxy-2',4',6'-triisopropyl-1,1'-biphenyl. | [References]
[1] Journal of the American Chemical Society, 2008, vol. 130, # 41, p. 13552 - 13554
[2] Patent: WO2009/76622, 2009, A2. Location in patent: Page/Page column 115-116 |
| Questions And Answer | Back Directory | [Reactions]
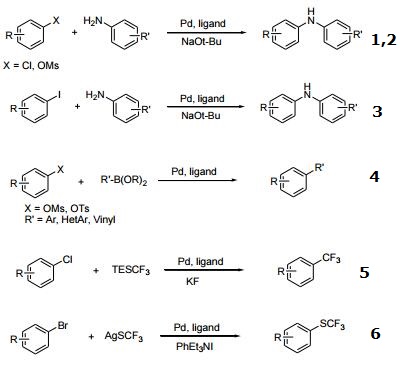
- Ligand for palladium-catalyzed cross-coupling reactions using aryl mesylates with electron-deficient anilines.
- Ligand for palladium-catalyzed cross-coupling of primary arylamines at low catalyst loading.
- Ligand for palladium-catalyzed cross-coupling of aryl iodides and primary amines.
- Ligand for the Suziki-Miyaura coupling of tosylates and mesylates.
- Ligand for the palladium-catalyzed trifluoromethylation of aryl chlorides.
- Ligand for the palladium-catalyzed formation of aryl-SCF3 compounds from aryl bromides.
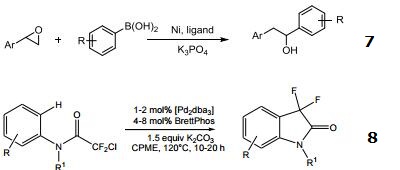
- Ligand for the nickel-catalyzed cross-coupling of styrenyl epoxides with boronic acids.
- Ligand for the palladium-catalyzed intramolecular CH difluoroalkylation.
|
|
|