| Identification | More | [Name]
2-(Dicyclohexylphosphino)biphenyl | [CAS]
247940-06-3 | [Synonyms]
(2-BIPHENYLYL)DICYCLOHEXYLPHOSPHINE
2-(DICYCLOHEXYLPHOSPHINO)BIPHENYL
2-(DICYLOHEXYLPHOSPHINO)BIPHENYL
CYCLOHEXYL JOHNPHOS
2-(Dicyclohexylphosphino)biphenyl2-(Dicyclohexylphosphino)biphenyl
2-(Dicyclohexylphosphino)biphenyl,98%
(2-biphenyl)dicyclohexylphosphine
2-(DICYCLOHEXYLPHOSPHINO)BIPHENYL 98%
PHOSPHINE, [1,1''-BIPHENYL]-2-YLDICYCLOHEXYL-
2-(Dicyclohexylphosphino)biphenyl, Cyclohexyl JohnPhos | [EINECS(EC#)]
480-030-2 | [Molecular Formula]
C24H31P | [MDL Number]
MFCD01862441 | [Molecular Weight]
350.48 | [MOL File]
247940-06-3.mol |
| Chemical Properties | Back Directory | [Appearance]
white to light yellow crystal powde | [Melting point ]
102-106 °C(lit.)
| [Boiling point ]
499.5±24.0 °C(Predicted) | [storage temp. ]
Inert atmosphere,2-8°C | [solubility ]
soluble in Toluene | [form ]
crystal | [color ]
white | [Sensitive ]
Air Sensitive | [Usage]
Ligand employed in an extremely general method for the Pd-catalyzed synthesis of aromaticamines using aryl chlorides, bromides and triflates. | [BRN ]
8440533 | [InChI]
InChI=1S/C24H31P/c1-4-12-20(13-5-1)23-18-10-11-19-24(23)25(21-14-6-2-7-15-21)22-16-8-3-9-17-22/h1,4-5,10-13,18-19,21-22H,2-3,6-9,14-17H2 | [InChIKey]
LCSNDSFWVKMJCT-UHFFFAOYSA-N | [SMILES]
P(C1=CC=CC=C1C1=CC=CC=C1)(C1CCCCC1)C1CCCCC1 | [CAS DataBase Reference]
247940-06-3(CAS DataBase Reference) | [Storage Precautions]
Moisture sensitive;Store under inert gas;Air sensitive |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R22:Harmful if swallowed. | [Safety Statements ]
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
3
| [F ]
10-13-23 | [TSCA ]
No | [HazardClass ]
AIR SENSITIVE | [HS Code ]
29310099 | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Aquatic Chronic 4 |
| Hazard Information | Back Directory | [Description]
2-(Dicyclohexylphosphino)biphenyl is a ligand for the amination of triflates and aryl halides. Optimal ligand for a novel amination reaction (Buchwald reaction)(2) 2-(Dicyclohexylphosphino)biphenyl, a synthetic chemical compound, has found diverse applications in scientific research. Being a cyclic phosphonate derivative of cyclohexanol, this colourless, odourless, and tasteless solid holds a distinct appeal for laboratory use. In scientific research, 2-(Dicyclohexylphosphino)biphenyl has proven invaluable. It serves as an excellent model compound for delving into protein-ligand interactions, thus aiding in studying small molecule effects on protein folding. Moreover, researchers have utilized 2-(Dicyclohexylphosphino)biphenyl to gain insights into protein structures and unravel the mechanism of action behind various drugs. Additionally, the compound has been a vital tool in assessing how environmental factors influence protein structure and function. The interaction between 2-(Dicyclohexylphosphino)biphenyl and proteins primarily occurs through hydrogen bonding and hydrophobic interactions, resulting in high-affinity and specific protein binding. | [Chemical Properties]
white to light yellow crystal powde | [Uses]
Ligand employed in an extremely general method for the Pd-catalyzed synthesis of aromaticamines using aryl chlorides, bromides and triflates. | [Uses]
suzuki reaction | [General Description]
CyJohnPhos [(2-Biphenyl)dicyclohexylphosphine] is an air-stable, bulky and electron-rich monodentate biarylphosphine ligand developed by the Buchwald group to enhance the reactivity of palladium catalysis during cross-coupling reactions. | [reaction suitability]
reaction type: Cross Couplings
reagent type: ligand
reaction type: Arylations
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: C-X Bond Formation
reagent type: ligand
reaction type: Hiyama Coupling
reagent type: ligand
reaction type: Methylations
reagent type: ligand
reaction type: Negishi Coupling
reagent type: ligand
reaction type: Oxidations
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling | [Synthesis]
General procedure for the synthesis of 2-(dicyclohexylphosphino)biphenyl from 2-bromobiphenyl and dicyclohexylphosphine: under nitrogen protection, a mixture of L1 (0.05 or 0.10 mmol P), [PdCl(η3-C3H5)]2 (0.025 mmol), 2-bromobiphenyl (0.55 mmol) and dicyclohexylphosphine (0.50 mmol) was reacted in a 20 M KOH The mixture in aqueous solution (0.5-1.0 mL) was reacted by shaking at 100 °C for several hours. After completion of the reaction, the mixture was cooled to room temperature and filtered. The aqueous filtrate was extracted with degassed toluene (2 mL x 2 times). For the recovered catalyst resin beads, extract with degassed toluene (2 mL x 4 times). All extracts were combined and dried with anhydrous Na2SO4, followed by concentration under vacuum to obtain the crude product. The crude product was purified by silica gel fast chromatography using degassed eluent (n-hexane/Et2O = 10/0-9/1), and the purification process was completed by a YAMAZEN medium-pressure liquid chromatography system, which ultimately yielded the target product 2-(dicyclohexylphosphino)biphenyl. | [References]
[1] Synlett, 2017, vol. 28, # 20, p. 2966 - 2970 |
| Questions And Answer | Back Directory | [Reaction]
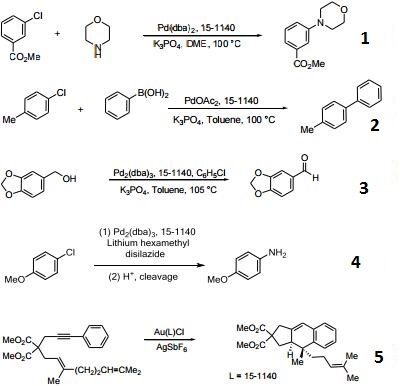
- Ligand used in the palladium-catalyzed synthesis of aromatic amines from aryl chlorides, bromides and triflates.
- Ligand employed in Suzuki coupling reactions involving aryl chlorides, bromides and triflates.
- Useful ligand for the Pd-catalyzed oxidation of alcohols in the presence of chlorobenzenes.
- Useful ligand for the Pd-catalyzed amination with ammonia equivalents.
- Ligand for the gold(I)-catalyzed intramolecular [4+2] cycloadditions involving 1,3-enynes and arylalkynes with alkenes.
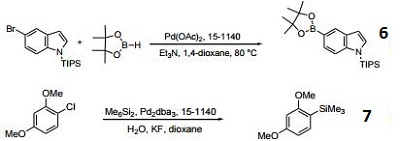
- Ligand used in the palladium-catalyzed borylation of aryl bromdies.
- Ligand used in the palladium-catalyzed siliylation of aryl chlorides.
|
|
|