| Identification | More | [Name]
Primidone | [CAS]
125-33-7 | [Synonyms]
2-deoxyphenobarbital
2-DESOXYPHENOBARBITAL
5-ETHYLDIHYDRO-5-PHENYL-4,6(1H,5H)-PYRIMIDINEDIONE
5-phenyl-5-ethyl-hexahydropyrimidine-4,6-dione
PRIMIDONE
4,6(1H,5H)-Pyrimidinedione, 5-ethyldihydro-5-phenyl-
5-Aethyl-5-phenyl-hexahydropyrimidin-4,6-dion
5-Ethyl-5-phenyldihydro-4,6(1H,5H)-pyrimidinedione
5-Ethyl-5-phenylhexahydropyrimidine-4,6-dione
5-ethylhexahydro-4,6-dioxo-5-phenylphrimidine
5-Ethylhexahydro-4,6-dioxo-5-phenylpyrimidine
5-Ethylhexahydro-5-phenylpyrimidine-4,6-dione
5h)-pyrimidinedione,5-ethyldihydro-5-phenyl-6(1h
5-Phenyl-5-aethylhexahydropyrimidindion-(4,6)
Cyral
Desoxyphenobarbitone
Hexadiona
Hexamidine
hexamidine(theantispasmodic)
Lepimidin | [EINECS(EC#)]
204-737-0 | [Molecular Formula]
C12H14N2O2 | [MDL Number]
MFCD00038662 | [Molecular Weight]
218.25 | [MOL File]
125-33-7.mol |
| Chemical Properties | Back Directory | [Appearance]
Crystalline Solid | [Melting point ]
281-282°C | [Boiling point ]
358.94°C (rough estimate) | [density ]
1.1402 (rough estimate) | [refractive index ]
1.6660 (estimate) | [Fp ]
9℃ | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Very slightly soluble in water, slightly soluble in ethanol (96 per cent). It dissolves in alkaline solutions. | [form ]
neat | [pka]
12.26±0.40(Predicted) | [color ]
White to Off-White | [Water Solubility ]
<0.1 g/100 mL at 19 ºC | [Usage]
Anticonvulsant | [Merck ]
14,7746 | [BCS Class]
2 | [InChIKey]
DQMZLTXERSFNPB-UHFFFAOYSA-N | [CAS DataBase Reference]
125-33-7(CAS DataBase Reference) | [IARC]
2B (Vol. 108) 2016 | [NIST Chemistry Reference]
Primidone(125-33-7) | [EPA Substance Registry System]
125-33-7(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed.
R40:Limited evidence of a carcinogenic effect. | [Safety Statements ]
S22:Do not breathe dust .
S36:Wear suitable protective clothing .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
3249 | [WGK Germany ]
3
| [RTECS ]
UV9100000
| [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
29335990 | [Safety Profile]
Poison by ingestion and
intraperitoneal routes. Human teratogenic
effects include developmental abnormalities
of the craniofacial area, skin and skin
appendages, and cardlovascular system.
Human reproductive effects: effects on
newborn, including unusual growth
statistics, drug dependence, physical and
other neonatal changes. Experimental
teratogenic and reproductive effects. Human
mutation data reported. An addictive drug.
When heated to decomposition it emits
toxic fumes of NOx. See also
BARBITURATES. | [Hazardous Substances Data]
125-33-7(Hazardous Substances Data) | [Toxicity]
child,TDLo,oral,625mg/kg (625mg/kg),BEHAVIORAL: SLEEPBEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY)BEHAVIORAL: GENERAL ANESTHETIC,British Medical Journal. Vol. 1, Pg. 90, 1957. |
| Hazard Information | Back Directory | [General Description]
Odorless white crystalline powder. Slightly bitter taste. No acidic properties. | [Reactivity Profile]
PRIMACLONE(125-33-7) is an amide. May react with azo and diazo compounds to generate toxic gases. May react with strong reducing agents to form flammable gases. A very weak base. The Combustion generates toxic mixed oxides of nitrogen (NOx). | [Air & Water Reactions]
Insoluble in water. | [Fire Hazard]
Flash point data for this chemical are not available; however, PRIMACLONE is probably combustible. | [Description]
Primidone is chemically and structurally similar to phenobarbital with the exception that the
carbonyl group on C2 is replaced by a methylene group. This modification leads to the pro�duction of a drug with strong anticonvulsant properties without expressed soporific effects. | [Chemical Properties]
Crystalline Solid | [Uses]
Anticonvulsant | [Uses]
Primidone is mainly used for major attacks. | [Definition]
ChEBI: A pyrimidone that is dihydropyrimidine-4,6(1H,5H)-dione substituted by an ethyl and a phenyl group at position 5. It is used as an anticonvulsant for treatment of various types of seizures. | [Brand name]
Mysoline (Valeant); Mysoline (Xcel). | [Biological Activity]
Anticonvulsant. | [Pharmacokinetics]
Approximately 60 to 80% of an oral dose of primidone is absorbed and slowly metabolized by the liver to phenobarbital and phenylethylmalonamide (PEMA). All three molecules have antiseizure effects, but PEMA appears to be weaker and to
be the more toxic metabolite. During chronic therapy, approximately 15 to 25% of an oral dose of primidone is excreted in the
urine unchanged, 15 to 25% metabolized to phenobarbital, and 50 to 70% excreted as PEMA (half-life, 24–48 hours). The
phenobarbital metabolite may be excreted in the urine unchanged, as its p-hydroxy metabolite, and as glucuronide or sulfate
conjugates. Following an oral dose, the peak plasma levels for primidone are reached in approximately 4 hours, with a reported
half-life of 10 to 12 hours. Plasma concentrations in the range of 8 to 12 μg/mL control seizures and minimize adverse effects.
Primidone shows antiseizure activity before the phenobarbital levels reach therapeutic range. Only after chronic dosing of
primidone are the levels of phenobarbital significant, suggesting autoinduction. Serum levels of chronically administered
primidone exceed those of its metabolite, phenobarbital, thus demonstrating that it has antiseizure activity independent of
phenobarbital. When primidone is coadministered with enzyme-inducing AEDs, the levels of its phenobarbital metabolite may be
two- to threefold higher than those in the noninduced state. Protein binding of primidone and PEMA is negligible, and the
phenobarbital metabolite is approximately 50% protein bound.
Primidone use is associated with decreases in CBZ, lamotrigine, valproate, tiagabine, and zonisamide serum levels. Primidone
levels are increased by nicotinamide and isoniazid. Hydantoins increase the plasma concentrations of primidone, phenobarbital,
and PEMA. CBZ increases levels of phenobarbital derived from primidone. Primidone levels are decreased by succinimides,
CBZ, and acetazolamide. | [Clinical Use]
Primidone is the 2-deoxy derivative of phenobarbital and is approved by the U.S. FDA for initial or adjunctive
treatment of simple partial, complex partial, and tonic-clonic seizures. It is less effective against these types of seizures than is
phenytoin or CBZ, and it shares the antiseizure and sedative actions of phenobarbital. Although not approved for the purpose, it often is
used to treat benign familial tremor (essential tremor). | [Side effects]
As with phenobarbital, serious toxicity for primidone is rare, although it may cause disabling sedation, irritability, and
decreased mental functioning in a number of persons. Ataxia, dysphoria, idiosyncratic rash, leukopenia, agranulocytosis,
lymphadenopathy, hepatitis, and a systemic lupus erythematosus–like syndrome have been reported adverse effects for
primidone. Deficiencies of folic acid and of vitamins D and K are possible with long-term therapy of primidone, as is a folate�responsive megaloblastic anemia. Measurement of the complete blood cell count should be performed at 6-month intervals. | [Synthesis]
Primidone, 5-ethyl-5-phenylhexahydropyrimidinedione-4,6 (9.2.1) is synthe�sized by reacting ethylphenylmalonic acid diamide with formamide [5,6]. An alternative
method is the electrolytic reduction of phenobarbital or the catalytic reduction of the
appropriate 2-thiobarbituric acid [7].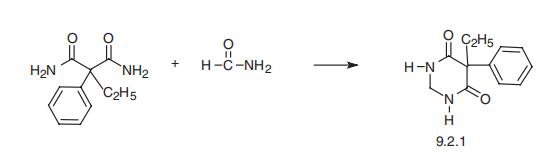
| [Veterinary Drugs and Treatments]
Primidone is indicated for seizure control (idiopathic epilepsy, epileptiform
convulsions) in the dog. Because it is rapidly converted
into phenobarbital in this species (see Pharmacokinetics below),
and has a greater incidence of hepatotoxicity and behavioral effects,
most neurologists do not recommend its use. However, some
clinicians feel that some animals not responding to phenobarbital
do benefit from primidone therapy, perhaps as a result that PEMA
has been demonstrated to potentiate the anticonvulsant activity
of phenobarbital in animals. When compared with phenobarbital,
increased incidence of hepatotoxicity associated with primidone
is considered the major limitation to long-term therapy with this
agent. Primidone is considered more toxic in rabbits and cats than
in humans or dogs. | [Drug interactions]
Potentially hazardous interactions with other drugs
Aminophylline and theophylline: metabolism of
aminophylline and theophylline increased, reduced
effect.
Anthelmintics: concentration of albendazole and
praziquantel reduced.
Anti-arrhythmics: reduced concentration of
disopyramide and possibly propafenone; possibly
reduced concentration of dronedarone - avoid.
Antibacterials: reduced concentration of
chloramphenicol, doxycycline, metronidazole,
telithromycin and rifampicin - avoid with
telithromycin.
Anticoagulants: increased metabolism of coumarins
(reduced effect); possibly reduced concentration of
apixaban and edoxaban and possibly rivaroxaban.
Antidepressants: antagonise anticonvulsant effect;
reduces concentration of paroxetine, reboxetine,
mianserin and tricyclics; concentration reduced by St
John’s wort - avoid.
Antiepileptics: concentration increased by
fosphenytoin, oxcarbazepine, phenytoin, stripentol
and valproate and possibly carbamazepine, also active
metabolite of oxcarbazepine reduced and valproate
concentration reduced, concentration of fosphenytoin
and phenytoin usually reduced but can also be
increased; concentration of ethosuximide, rufinamide
and topiramate possibly reduced; concentration of
lamotrigine, tiagabine and zonisamide reduced.
Antifungals: possibly reduced concentration
of isavuconazole, itraconazole, posaconazole
and voriconazole - avoid concomitant use with
voriconazole; reduced absorption of griseofulvin
(reduced effect).
Antimalarials: avoid with piperaquine with
artenimol; anticonvulsant effect antagonised by
mefloquine
Antipsychotics: antagonise anticonvulsant effect;
metabolism of haloperidol increased; possibly
reduces aripiprazole concentration - increase
aripiprazole dose; concentration of both drugs
reduced with chlorpromazine; possibly reduces
clozapine concentration; possibly reduces lurasidone
concentration - avoid.
Antivirals: concentration of abacavir, boceprevir,
darunavir, dolutegravir, fosamprenavir, indinavir,
lopinavir, rilpivirine and saquinavir possibly reduced;
avoid with boceprevir and rilpivirine; concentration
of daclatasvir, dasabuvir, ombitasvir, paritaprevir
and simeprevir possibly reduced - avoid; avoid with
elvitegravir, etravirine, ledipasvir, sofosbuvir and
telaprevir.
Calcium-channel blockers: effects of calcium-channel
blockers probably reduced - avoid with isradipine
and nimodipine.
Cannabis extract: concentration possibly reduced by
primidone - avoid.
Ciclosporin: reduced ciclosporin levels.
Cobicistat: concentration of cobicistat possibly
reduced.
Corticosteroids: metabolism of corticosteroids
accelerated, reduced effect.
Cytotoxics: possibly reduced concentration
of axitinib, increase axitinib dose; possibly
reduced concentration of bortezomib, bosutinib,
cabozantinib, ceritinib, crizotinib, dasatinib,
ponatinib and vandetanib - avoid; avoid with
cabazitaxel, dabrafenib, gefitinib and panobinostat;
concentration of irinotecan and its active metabolite
and possibly etoposide reduced; possible increased
hypersensitivity reactions with procarbazine.
Diuretics: concentration of eplerenone reduced -
avoid; increased risk of osteomalacia with carbonic
anhydrase inhibitors.
Guanfacine: concentration of guanfacine possibly
reduced - increase dose of guanfacine.
Hormone antagonists: possibly reduced
concentration of abiraterone - avoid; metabolism of
toremifene accelerated. | [Metabolism]
Partially metabolised to phenobarbital and
phenylethylmalonamide in the liver, both of which are
active and have longer half-lives compared to primidone
(metabolites may accumulate in renal impairment).
It is excreted in urine as unchanged drug and metabolites. | [storage]
Store at -20°C | [References]
[1] NORTON P R E. The effects of drugs on barbiturate withdrawal convulsions in the rat[J]. Journal of Pharmacy and Pharmacology, 1970, 22 10: 763-766. DOI: 10.1111/j.2042-7158.1970.tb08425.x |
|
|