| Identification | More | [Name]
Rimonabant | [CAS]
168273-06-1 | [Synonyms]
ACOMPLIA
RIMONABANT
RIMONABANT(ACOMPLIA,SR141716)
5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-piperidinopyrazole-3-carboxamide | [EINECS(EC#)]
200-223-5 | [Molecular Formula]
C22H21Cl3N4O | [MDL Number]
MFCD04034714 | [Molecular Weight]
463.79 | [MOL File]
168273-06-1.mol |
| Chemical Properties | Back Directory | [Melting point ]
154.7 °C | [density ]
1.41±0.1 g/cm3(Predicted) | [storage temp. ]
RT | [solubility ]
Soluble in DMSO (up to 20 mg/ml) or in Ethanol (up to 20 mg/ml). | [form ]
solid | [pka]
11.31±0.20(Predicted) | [color ]
White | [Stability:]
Stable for 1 year from date of purchase as supplied. Protect from moisture. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. | [InChI]
InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | [InChIKey]
JZCPYUJPEARBJL-UHFFFAOYSA-N | [SMILES]
N1(C2=CC=C(Cl)C=C2Cl)C(C2=CC=C(Cl)C=C2)=C(C)C(C(NN2CCCCC2)=O)=N1 | [CAS DataBase Reference]
168273-06-1(CAS DataBase Reference) |
| Questions And Answer | Back Directory | [Description]
Rimonabant is an inverse antagonist for the cannabinoid receptor (CB1). It acts by selectively blocking CB1 receptors found in the brain and in peripheral organs important in glucose and lipid metabolism, including adipose tissue, the liver, gastrointestinal tract, and muscle. Thus, rimonabant constitutes a therapeutic approach to obesity and cardiovascular risk factors. As an anorectic antiobesity drug, it was used as an adjunct to diet and exercise for obese or overweight patients with associated risk factors in Europe in 2006. Nevertheless, adverse effects including suicidality, depression, and anxiety were reported, based on which rimonadant was withdrawn worldwide in 2008.
Andogenous cannabinoids are related to the pleasurable effect of nicotine, rimonabant, as a cannabinoid receptor blocker, is also being tested as a potential anti-smoking treatment.
| [References]
[1] https://www.drugs.com/acomplia.html
[2] Leite CE, Mocelin CA, Petersen GO, Leal MB, Thiesen FV (2009) Rimonabant: an antagonist drug of the endocannabinoid system for the treatment of obesity, Pharmacol Rep., 61, 217-224
|
| Hazard Information | Back Directory | [Originator]
Sanofi-Synthelabo (France) | [Uses]
Rimonabant is a selective antagonist of CB1 with IC50 of 13.6 nM and EC50 of 17.3 nM in hCB1 transfected HEK 293 membrane | [Definition]
ChEBI: Rimonabant is a carbohydrazide obtained by formal condensation of the carboxy group of 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxylic acid with the amino group of 1-aminopiperidine. It is a potent and selective cannabinoid receptor 1 (CB1R) antagonist. Besides its antagonistic properties, numerous studies have shown that, at micromolar concentrations rimonabant behaves as an inverse agonist at CB1 receptors. The drug was the first selective CB1 antagonist/inverse agonist introduced into clinical practice to treat obesity and metabolic-related disorders. It was later withdrawn from market due to CNS-related adverse effects including depression and suicidal ideation. It has a role as an anti-obesity agent, a CB1 receptor antagonist and an appetite depressant. It is a member of pyrazoles, a dichlorobenzene, a carbohydrazide, an amidopiperidine and a member of monochlorobenzenes. | [Brand name]
Acomplia (Sanofi-Synthe-labo). | [Synthesis]
The reported preparation of rimonabant,
both in small and large scale, is shown in the scheme. Lithium enolate formation of p-chlorophenyl ethyl ketone 54 with LiHMDS in THF at -78oC for 45 min followed
by reaction with diethyl oxalate at -78oC and warming
to room temperature over 16 h provided the lithium enolate
salt of the diketoester 55. Reaction of diketoester salt 55 with
2,4-dichlorophenyl hydrazine (56) in ethanol at room temperature
gave intermediate hydrazone 57 which is then cyclized
in refluxing acetic acid for 24 h to obtain pyrazole
ester 58. Hydrolysis of ester 58 with KOH in refluxing
methanol:water mixture gave acid 59 which was then converted
to the acid chloride 60 with thionyl chloride in refluxing
toluene in very good yield. On scale, the synthesis of the
acid chloride was performed in cyclohexane at 83oC. Reaction
of acid chloride 60 with 1-aminopiperidine (61) in the
presence of triethylamine at 0oC to room temperature over 3h
gave rimonabant (VIII) which was isolated as the HCl salt
by treating it with HCl in ether.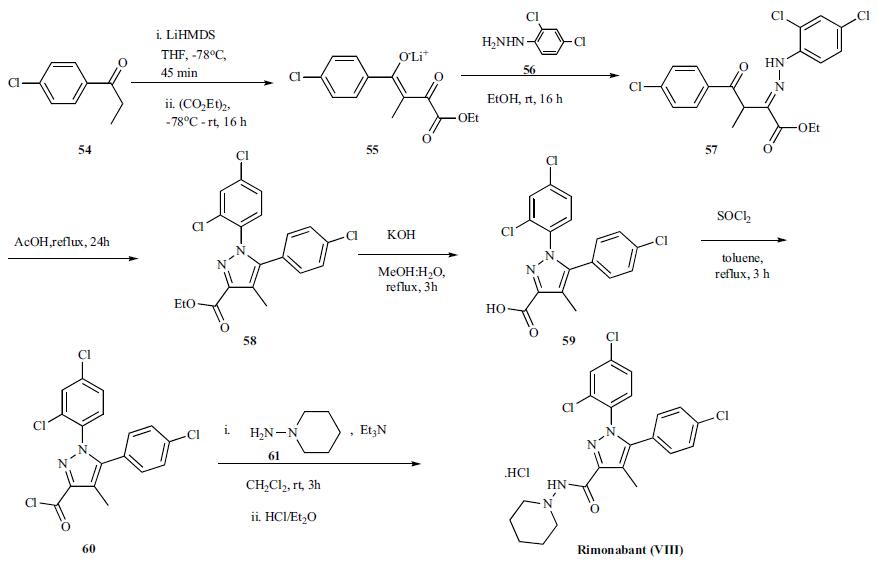
|
|
|