| Identification | More | [Name]
Bis-(3-phthalyl anhydride) ether | [CAS]
1823-59-2 | [Synonyms]
4-4'-OXYDIPHTHALIC ACID ANHYDRIDE
4,4'-OXYDIPHTHALIC ANHYDRIDE
BIS-(3-PHTHALYL ANHYDRIDE) ETHER
ODPA
4,4'-Oxydiphthalic dianhydride
5,5’-oxybis-3-isobenzofurandione
oxydiphthalicanhydride
4,4'-Oxydiphthalic Acid
4,4'-OXYDIPHTHALIC ANHYDRIDE 97%
4,4''-OXYBISDIPHTHALIC ANHYDRIDE
5,5'-oxybis-1,3-isobenzofurandione
3,3',4,4'-Diphenylether tetracarboxylic dianhydride
4,4'-Oxybis(1,2-benzenedicarboxylic acid)1,2:1',2'-dianhydride
4,4'-Oxybis(benzene-1,2-dicarboxylic acid)1,2:1',2'-dianhydride
4,4'-Oxybis(phthalic acid)1,2:1',2'-dianhydride
4,4'-Oxybisphthalic 1,2:1',2'-dianhydride
4,4'-Oxybisphthalic acid-1,2:1',2'-dianhydride
4,4'-oxydiphthalic Anhydride(ODPA) | [EINECS(EC#)]
412-830-4 | [Molecular Formula]
C16H6O7 | [MDL Number]
MFCD00039144 | [Molecular Weight]
310.21 | [MOL File]
1823-59-2.mol |
| Questions And Answer | Back Directory | [Description]
4,4'-Oxydiphthalic anhydride/ODPA(CAS:1823-59-2) is a solid, melts in the range of 225-229 °C and was first introduced in the mid-1980s, offering a greater degree of flexibility than other commercial dianhydrides. It also is somewhat less reactive than BTDA. ODPA has largely been used in polyimides and much less so in epoxies. It imparts special performance properties in free films and in FCCL production for high-end personal electronic devices.
|
| Chemical Properties | Back Directory | [Appearance]
off-white to white powder | [Melting point ]
225-229 °C(lit.)
| [Boiling point ]
410.39°C (rough estimate) | [density ]
1.4942 (rough estimate) | [refractive index ]
1.6380 (estimate) | [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
very slightly in Toluene,Acetone | [form ]
powder to crystal | [color ]
White to Light yellow to Light orange | [InChI]
InChI=1S/C16H6O7/c17-13-9-3-1-7(5-11(9)15(19)22-13)21-8-2-4-10-12(6-8)16(20)23-14(10)18/h1-6H | [InChIKey]
QQGYZOYWNCKGEK-UHFFFAOYSA-N | [SMILES]
O(C1C=CC2C(=O)OC(=O)C=2C=1)C1C=CC2C(=O)OC(=O)C=2C=1 | [CAS DataBase Reference]
1823-59-2(CAS DataBase Reference) | [NIST Chemistry Reference]
Bis-(3-phthalyl anhydride) ether(1823-59-2) | [EPA Substance Registry System]
1823-59-2(EPA Substance) |
| Safety Data | Back Directory | [Risk Statements ]
R52/53:Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment . | [Safety Statements ]
S61:Avoid release to the environment. Refer to special instructions safety data sheet . | [WGK Germany ]
2
| [TSCA ]
TSCA listed | [HS Code ]
29189900 | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Aquatic Chronic 3 |
| Hazard Information | Back Directory | [Chemical Properties]
off-white to white powder | [Uses]
4,4'-oxydiphthalic anhydride(1823-59-2) be used for the preparation of a polyimide material.
| [Preparation]
The preparation of 4,4'-Oxydiphthalic anhydride(1823-59-2) is as follows:Chlorophthalic anhydride (30g, 160mmol), tetraphenyl phosphonium bromide (0.3g, 0.7mmol), and 2,5-dichlorobenzoic acid (0.08g, 0.4mmol) were heated to approximately 220℃. Potassium carbonate (7.95g, 58mmol) was added over 45 minutes. The reaction was sampled after addition of the potassium carbonate and showed a 24.9% conversion to 4,4'-oxydiphthalic anhydride. The reaction was heated for an additional 45 minutes. The reaction mixture was diluted with 1,2,4-trichlorobenzene (90g) and filtered. The 4,4'-oxydiphthalic anhydride was allowed to crystallize and was collected, washed with 1,2,4-trichlorobenzene followed by hexane, and dried in an air circulating oven at 120℃. to give crude 4,4'-oxydiphthalic anhydride, 12.9g.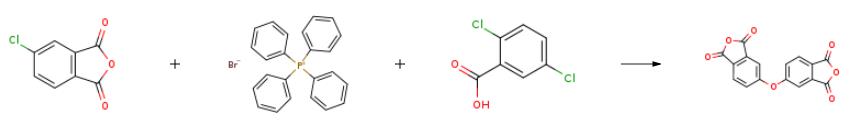
| [Flammability and Explosibility]
Notclassified | [Synthesis]
The general procedure for the synthesis of 4,4'-oxybisphthalic anhydride from 4-chlorophthalic anhydride is as follows:
1. 4-chlorophthalic anhydride (16 g, 87.7 mmol) was added to a 150 ml three-necked flask fitted with a distillation head, which was pre-filled with about 100 ml of distilled o-dichlorobenzene containing less than 5 ppm of water.
2. The mixture was heated to reflux for 0.5 h under nitrogen protection, followed by distillation to remove about 80 ml of o-dichlorobenzene.
3. To another flask was added powdered potassium carbonate (6.06 g, 43.8 mmol) and 50 ml of anhydrous o-dichlorobenzene.
4. The suspension was heated to reflux for 0.5 h under nitrogen protection and 40 ml of o-dichlorobenzene was distilled.
5. The 4-chlorophthalic anhydride solution prepared in step 1 was transferred to a flask containing potassium carbonate.
6. The mixture was stirred, hexaethylguanidine chloride (468 mg, 1.77 mmol, as an 18% solution in o-dichlorobenzene containing less than 15 ppm of water) was added and refluxing was continued, observing a gradual change in color of the solution to yellow.
7. The mixture was analyzed periodically and 93% yield of 4,4'-oxybisphthalic anhydride was achieved after 3 hours.
8. in a control experiment using the same molar percentage of tetraphenylphosphonium bromide and powdered potassium carbonate it took 13 hours to achieve the same yield.
9. Repeating the experiment, switching to granular potassium carbonate and adjusting the amount of hexaethylguanidine chloride to 2.0 mol% of 4-chlorophthalic anhydride, the product formation was stabilized after 3 hours in an overall yield of about 85% or better.
Note: The above description and embodiments are for illustrative purposes only and should not be regarded as a limitation of the present invention. Various modifications, adaptations and substitutions may be made by those skilled in the art without departing from the spirit and scope of the present invention. | [IC 50]
HepG2: IC50 = 121 μM (human); SK-HEP1: IC50 = 199 μM (human); Hepatocyte: IC50 >200 μM (rat) | [References]
[1] Patent: US6706897, 2004, B1. Location in patent: Page column 3-4
[2] Patent: CN108250168, 2018, A. Location in patent: Paragraph 0009-0010; 0011-0012; 0013-0014; 0015-0016
[3] Patent: US6727370, 2004, B1. Location in patent: Page column 4
[4] Patent: US6727370, 2004, B1. Location in patent: Page column 4
[5] Patent: US2007/117990, 2007, A1. Location in patent: Page/Page column 12-13 |
|
|