| Identification | Back Directory | [Name]
Prazosin | [CAS]
19216-56-9 | [Synonyms]
pressin
PRAZOSIN
minipress
furazosin
AKOS B020009
PrazosinBase
Terazosin-010
Prazosin USP/EP/BP
ART-CHEM-BB B020009
Terazosin Impurity15
Terazosin EP Impurity K
Prazosin Hcl 19237-84-4 / Base
2-[4-(2-Furoyl)piperazin-1-yl]-6,7-dimethoxyquinazolin-4-amine
2-(4-(2-furoyl)piperazin-1-yl)-4-amino-6,7-dimethoxyquinazoline
4-amino-6,7-dimethoxy-2-(4-(2-furoyl)piperazin-1-yl)-quinazolin
1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furoyl)-piperazin
2-[4-(furan-2-carbonyl)piperazin-1-yl]-6,7-dimethoxyquinazolin-4-amine
1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl)-piperazin
1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl)piperazine
1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl)piperazine.
1-(4-Amino-6,7-dimethoxyquinazolin-2-yl)-4-(furan-2-ylcarbonyl)piperazine
1-(4-amino-6,7-dimethoxy-2-quinazolinyl-4-(2-furanylcarbonyl)) piperrazine
PIPERAZINE, 1-(4-AMINO-6,7-DIMETHOXY-2-QUINAZOLINYL)-4-(2-FURANYLCARBONYL)-
[4-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-1-piperazinyl]-2-furanyl-methanone
(2-Furanyl)[4-(4-amino-6,7-dimethoxyquinazoline-2-yl)piperazine-1-yl] ketone
[4-(4-amino-6,7-dimethoxy-quinazolin-2-yl)piperazin-1-yl]-(2-furyl)methanone
[4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl]-furan-2-ylmethanone
Methanone, [4-(4-amino-6,7-dimethoxy-2-quinazolinyl)-1-piperazinyl]-2-furanyl-
[4-(4-azanyl-6,7-dimethoxy-quinazolin-2-yl)piperazin-1-yl]-furan-2-yl-methanone
1-(4-aMino-6,7-diMethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl)piperazine hidrochloride
(4-(4-AMino-6,7-diMethoxyquinazolin-2-yl)piperazin-1-yl)(furan-2-yl)Methanone hydrochloride salt | [EINECS(EC#)]
242-885-8 | [Molecular Formula]
C19H21N5O4 | [MDL Number]
MFCD00599563 | [MOL File]
19216-56-9.mol | [Molecular Weight]
383.4 |
| Chemical Properties | Back Directory | [Melting point ]
278-280°C | [Boiling point ]
510.33°C (rough estimate) | [density ]
1.3275 (rough estimate) | [refractive index ]
1.7600 (estimate) | [storage temp. ]
Sealed in dry,2-8°C | [solubility ]
DMSO (Slightly), Methanol (Slightly, Heated) | [form ]
Solid | [pka]
pKa 6.54(50% EtOH) (Uncertain) | [color ]
White to Off-White | [Water Solubility ]
3.2mg/L(22.5 ºC) | [InChI]
InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | [InChIKey]
IENZQIKPVFGBNW-UHFFFAOYSA-N | [SMILES]
C(N1CCN(C2=NC(N)=C3C(=N2)C=C(OC)C(OC)=C3)CC1)(C1=CC=CO1)=O |
| Hazard Information | Back Directory | [Chemical Properties]
Crystallizes, melting point 278-280°C. Its hydrochloride ([19237-84-4]) is a white crystalline powder, melting point 275°C (decomposition). It is sparingly soluble in ethanol but slightly soluble in isotonic saline and water. It is odorless and tastes bitter. | [Originator]
Hypovase, Pfizer, UK,1974 | [History]
Prazosin is a drug used to treat hypertension. Prazosin is marketed by Pfizer and was initially approved by the FDA in 1988. It belongs to the class of drugs known as alpha-1 antagonists.
According to FDA, in October 2025, New Jersey-based drugmaker Teva Pharmaceuticals USA and drug distributor Amerisource Health Services issued voluntary nationwide recalls of over half a million bottles of various strengths of prazosine capsules, which may include a cancer-causing chemical. | [Uses]
Antihypertensive. | [Definition]
ChEBI: A member of the class of piperazines that is piperazine substituted by a furan-2-ylcarbonyl group and a 4-amino-6,7-dimethoxyquinazolin-2-yl group at positions 1 and 4 respectively. | [Manufacturing Process]
Preparation of 2-Chloro-4-Amino-6,7-Dimethoxyquinazoline: To 800 ml of a solution of anhydrous ammonia in tetrahydrofuran at room temperature is added 30 g of 2,4-dichloro-6,7-dimethoxyquinazoline [F.H.S. Curd et al., J. Chem. Soc., p 1759 (1948)]. The mixture is stirred for 44 hours. The precipitate (29 g, MP 267° to 268°C) is filtered and recrystallized from methanol to yield 19 g of 2-chloro-4-amino-6,7-dimethoxyquinazoline, MP 302°C (dec.).
Preparation of 2-(1-Piperazinyl)-4-Amino-6,7-Dimethoxyquinazoline: To 5 g of 2-chloro-4-amino-6,7-dimethoxyquinazoline, is added 20 g of a 25% solution of piperazine in ethanol. The mixture is heated at 160°C for 16 hours in a pressure bottle. The solvent is then evaporated and the residue is recrystallized from methanol/water.
Preparation of 2[4-(2-Furoyl)-Piperazinyl]-4-Amino-6,7-Dimethoxyquinazoline: To 0.10 mol 2-(1-piperazinyl)-4-amino-6,7-dimethoxyquinazoline in 300 ml methanol is added with vigorous stirring, 0.10 mol 2-furoyl chloride. After addition is complete, the mixture is stirred for 3 hours at room temperature. The solids are filtered to give the desired product, MP 278° to 280°C. | [Brand name]
Minipress
(Pfizer). | [Therapeutic Function]
Antihypertensive | [Clinical Use]
Prazosin is effective in reducing all grades of hypertension.
The drug can be administered alone in mild
and (in some instances) moderate hypertension.When
the hypertension is moderate or severe, prazosin generally
is given in combination with a thiazide diuretic
and a -blocker. The antihypertensive actions of prazosin
are considerably potentiated by coadministration
of thiazides or other types of antihypertensive
drugs.
Prazosin may be particularly useful when patients
cannot tolerate other classes of antihypertensive drugs
or when blood pressure is not well controlled by other
drugs. Since prazosin does not significantly influence
blood uric acid or glucose levels, it can be used in hypertensive patients whose condition is complicated by
diabetes mellitus or gout.
Prazosin and other -antagonists find use in the
management of benign prostatic obstruction, especially
in patients who are not candidates for surgery. Blockade
of -adrenoceptors in the base of the bladder and in the
prostate apparently reduces the symptoms of obstruction
and the urinary urgency that occurs at night. | [Side effects]
Although less of a problem than with phenoxybenzamine
or phentolamine, symptoms of postural hypotension,
such as dizziness and light-headedness, are the
most commonly reported side effects associated with
prazosin therapy. These effects occur most frequently
during initial treatment and when the dosage is sharply
increased. Postural hypotension seems to be more pronounced
during Na deficiency, as may occur in patients
on a low-salt diet or being treated with diuretics, -
blockers, or both. | [Synthesis]
Prazosin, 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furoyl)-piperazine
(12.2.12), is synthesized from 2-amino-4,5-dimethoxybenzoic acid, which upon reaction
with sodium cyanate undergoes heterocyclation into 2,4-dihydroxy-6,7-dimethoxyquina�zoline (12.2.9). Substituting hydroxyl groups of this compound with chlorine atoms by
reaction with thionyl chloride, or a mixture of phosphorous oxychloride with phosphorous
pentachloride gives 2,4-dichloro-6,7-dimethoxyquinazoline (12.2.10). Upon subsequent
reaction with ammonia, the chlorine atom at C4 of the pyrimidine ring is replaced with an
amino group, which leads to the formation of 4-amino-2-chloro-6,7-dimethoxyquinazoline
(12.2.11). Introducing this into a reaction with 1-(2-furoyl)piperazine gives prazosin
(12.2.12) [38¨C47].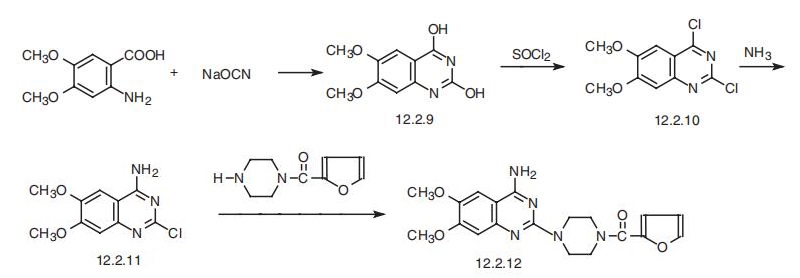
| [Veterinary Drugs and Treatments]
Prazosin is less well studied in dogs than hydralazine, and its capsule
dosage form makes it less convenient for dosing. Prazosin, however,
appears to have fewer problems with causing tachycardia, and its
venous dilation effects may be an advantage over hydralazine when
preload reduction is desired. It could be considered for therapy for
the adjunctive treatment of CHF, particularly when secondary to
mitral or aortic valve insufficiency when hydralazine is ineffective
or not tolerated. Prazosin may also be used for the treatment of
systemic hypertension or pulmonary hypertension in dogs. | [Drug interactions]
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Antidepressants: enhanced hypotensive effect with
MAOIs.
Avanafil, vardenafil, sildenafil and tadalafil: enhanced
hypotensive effect - avoid.
Beta-blockers: enhanced hypotensive effect, increased
risk of first dose hypotensive effect.
Calcium-channel blockers: enhanced hypotensive
effect, increased risk of first dose hypotensive effect.
Diuretics: enhanced hypotensive effect, increased risk
of first dose hypotensive effect.
Moxisylyte: possibly severe postural hypotension
when used in combination. | [Metabolism]
Prazosin is extensively metabolised in the liver, mainly by
demethylation and conjugation; some of the metabolites
have antihypertensive activity.
It is excreted as metabolites and 5-11% as unchanged
prazosin mainly in the faeces via the bile. |
|
|