| Identification | More | [Name]
Tinidazole | [CAS]
19387-91-8 | [Synonyms]
TIMTEC-BB SBB006917
TINIDAZOLE
1-(2-(ethylsulfonyl)ethyl)-2-methyl-5-nitro-1h-imidazol
1-(2-(ethylsulfonyl)ethyl)-2-methyl-5-nitro-imidazol
1-(2-(ethylsulfonyl)-ethyl)-2-methyl-5-nitroimidazole
bioshik
cp12574
ethyl(2-(2-methyl-5-nitro-1-imidazolyl)ethyl)sulfone
fasigin
fasigyn
pletil
simplotan
sorquetan
tinidazol
tricolam
TinidazoleTinidazoleBp
1H-Imidazole, 1-2-(ethylsulfonyl)ethyl-2-methyl-5-nitro-
TINIDAZOLE,USP
pleti
1-[2-(Ethylsulfonyl)ethyl]-2-methyl-5-nitro-1H-imidazole | [EINECS(EC#)]
243-014-4 | [Molecular Formula]
C8H13N3O4S | [MDL Number]
MFCD00057217 | [Molecular Weight]
247.27 | [MOL File]
19387-91-8.mol |
| Chemical Properties | Back Directory | [Appearance]
solid | [Melting point ]
118-120?C | [Boiling point ]
528.4±30.0 °C(Predicted) | [density ]
1.4338 (rough estimate) | [refractive index ]
1.6320 (estimate) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Practically insoluble in water, soluble in acetone and in methylene chloride, sparingly soluble in methanol. | [form ]
neat | [pka]
2.30±0.34(Predicted) | [color ]
Off-White to Pale Yellow | [Stability:]
Stable. Incompatible with strong oxidizing agents. | [λmax]
317nm(H2O)(lit.) | [Merck ]
14,9447 | [BRN ]
618182 | [Major Application]
forensics and toxicology
pharmaceutical (small molecule) | [InChI]
1S/C8H13N3O4S/c1-3-16(14,15)5-4-10-7(2)9-6-8(10)11(12)13/h6H,3-5H2,1-2H3 | [InChIKey]
HJLSLZFTEKNLFI-UHFFFAOYSA-N | [SMILES]
CCS(=O)(=O)CCn1c(C)ncc1[N+]([O-])=O | [CAS DataBase Reference]
19387-91-8(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R40:Limited evidence of a carcinogenic effect. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [RTECS ]
NI6255000
| [HS Code ]
2933290000 | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Acute Tox. 4 Dermal
Acute Tox. 4 Inhalation
Carc. 2
Muta. 2 | [Hazardous Substances Data]
19387-91-8(Hazardous Substances Data) | [Toxicity]
LD50 in mice (mg/kg): >3600 orally; >2000 i.p. (Miller) |
| Questions And Answer | Back Directory | [Pharmacology and mechanism of action]
Similar to metronidazole.
| [Pharmacology and mechanism of action]
Similar to metronidazole.
| [Indications]
Infections caused by Entamoeba histolytica and Giardia lamblia. Tinidazole is more effective than metronidazole in the treatment of giardiasis.
| [Side effects]
Side effects are similar to but milder than those caused by metronidazole. Gastrointestinal disturbances like nausea, vomiting, anorexia and metallic taste are common. Headache, tiredness, furred tongue and itching may occur. Thrombophlebitis may occur at the site of intravenous infusion [1].
| [Contraindications]
Tinidazole should not be taken together with alcohol.
| [Interactions]
A disulfiram-like reaction might occur if tinidazole is taken together with alcohol.
| [Preparations]
• Fasigyn® (Pfizer). Tablets 150 mg, 200 mg, 300 mg, 500 mg, 1 g. Oral suspension 200 mg per ml. Solution for injection 2 mg per ml.
• Tricolam® (Pfizer). Tablets 500 mg.
• Simplotan® (Pfizer). Tablets 1 g.
| [References]
1. Sawyer PR, Brogden RN, Pinder RM, Speight TM, Avery GS (1976). Tinidazole: a review of its antiprotozoal activity and therapeutic efficacy. Drugs, 11, 424–440.
|
| Hazard Information | Back Directory | [Chemical Properties]
solid | [Originator]
Simplotan,Pfizer,W. Germany,1971 | [Uses]
anticonvulsant | [Uses]
Antiprotozoal (Trichomonas, Giardia); antiamebic; antibacterial. | [Uses]
anti-ulcerative | [Uses]
For the treatment of trichomoniasis caused by T. vaginalis in both female and male patients. Also for the treatment of giardiasis caused by G. duodenalis in both adults and pediatric patients older than three years of age and for the treatment of intestin | [Definition]
ChEBI: Tinidazole is 1H-imidazole substituted at C-1 by a (2-ethylsulfonyl)ethyl group, at C-2 by a methyl group and at C-5 by a nitro group. It is used as an antiprotozoal, antibacterial agent. It has a role as an antiprotozoal drug, an antibacterial drug, an antiparasitic agent and an antiamoebic agent. | [Manufacturing Process]
The preparation of ethylsulfonylethyl-p-toluenesulfonate is carried out in the
following manner: 69.0 grams (0.5 mol) ethylsulfonylethanol dissolved in 150
ml pyridine is cooled to 0°C with stirring and while maintaining the
temperature between 0° to 10°C, 95 grams (0.5 mol) p-toluenesulfonyl
chloride is added in portions over a 10 minute period. After this time, 250 ml
water is added slowly and the mixture extracted with chloroform, the organic
phase washed first with 2 N HCl, then with water, separated and dried. The
product which crystallizes on cooling is filtered and dried to give 77.5% yield
of this intermediate.
A mixture of 12.7 grams (0.1 mol) of 2-methyl-5-nitroimidazole and 58.4
grams (0.2 mol) ethylsulfonylethyl-p-toluenesulfonate is heated with stirring,
under nitrogen, at 145° to 150°C for about 4 hours. After this time, the
reaction mixture is extracted with 500 ml hot water, the aqueous portion
adjusted with 10% Na2CO3 to a pH of 9 and extracted with chloroform (3
times with 150 ml portions). The separated organic phase is washed with
water, dried with Na2SO4 and evaporated to dryness. The crude tinidazole
product is then crystallized from benzene to give 4.36 grams of product
having a MP of 127° to 128°C. | [Therapeutic Function]
1-[2-(Ethylsulfonyl)ethyl]-2-methyl-5-nitroimidazole | [Antimicrobial activity]
Its antibacterial and antiprotozoal activity is similar to that of metronidazole.The MIC against G. vaginalis is 0.2–2 mg/L; the hydroxy metabolite is significantly more active than that of metronidazole. H. pylori is inhibited by 0.5 mg/L. T. vaginalis and T. fetus at 2.5 mg/L and E. histolytica is inhibited by about 0.3–2.5 mg/L. | [Pharmaceutical Applications]
A 5-nitroimidazole available for oral administration and, in some countries, for intravenous infusion. | [Mechanism of action]
Tinidazole has a mechanism of action that parallels that of metronidazole as well
as a similar metabolic pathway leading to hydroxylation at the 2-methyl group catalyzed by
CYP3A4. Basically, tinidazole appears to mimic the actions of metronidazole, although there are
reports that it is effective against some protozoa which are resistant to metronidazole. | [Pharmacokinetics]
Oral absorption :>95%
Cmax 2 g oral:40 mg/L after 2 h
800 mg (30-min infusion): 12 mg/L 6 min after end infusion
Plasma half-life: 12–14 h
Volume of distribution 0.64 L/kg :Plasma protein binding 12%
absorption and distribution
After a 2 g oral dose, concentrations remain at c. 10 mg/L at 24 h and 2.5 mg/L at 48 h. Daily doses of 1 g maintain plasma levels in excess of 8 mg/L, irrespective of whether the dose is oral or intravenous. It is well distributed, with concentrations in bile, CSF, breast milk and saliva similar to those reached in plasma.
The drug readily crosses the placenta. In women undergoing first trimester abortion, concentrations of 4.9 mg/kg (placenta) and 7.6 mg/kg (fetus) were found when the plasma concentration was 13.2 mg/L.
Metabolism and excretion
Metabolites include the 2-hydroxymethyl derivative, its glucuronide and two unidentified minor derivatives. In urine about half the drug remains unmetabolized.
The parent drug and its metabolites are excreted primarily in the urine and to a minor extent in the feces. The clearance rate is about 0.73 mL/min per kg and the urinary excretion is about 21% of the dose. Total clearance of the drug is 51 mL/min, renal clearance 10 mL/min. In healthy volunteers given an : intravenousinfusion of 800 mg [14C]tinidazole over 30 min, a mean of 44% of the dose was excreted in the urine during the first 24 h, increasing to 63% over 5 days: only 12% of the dose appeared in the feces. Unchanged tinidazole comprised 32% of urinary 14C in 0&ndash:12 h urine. The 2-hydroxymethyl metabolite accounted for about 9% of the urinary 14C and was also present in plasma.
In renal failure the pharmacokinetics are not significantly different from those in healthy individuals. It is rapidly removed by hemodialysis and a normal dose should be given after each dialysis: if treatment precedes dialysis a half dose should be infused after the end of the procedure. | [Clinical Use]
Anaerobic bacterial infections(prophylaxis and treatment)
Trichomoniasis
Giardiasis (single dose) Amebiasis (including amebic liver abscess)
Bacterial vaginosis
Gastric colonization with H. pylori (in combination with other agents) | [Synthesis]
Tinidazole, 1-2-(ethylsulfonyl)ethyl-2-methyl-5-nitroimidazole (37.2.12), is
also made from 2-methyl-5-nitroimidazole (37.2.9), which upon being reacted with
2-ethoxysulfonyl-p-toluenesulfonate (37.2.11) transformed into the desired tinidazole.
The 2-ethoxysulfonyl-p-toluenesulfonate (37.2.11) necessary for this reaction is in turn
made by tosylation of 2-ethylsulfonyl ethanol using p-toluenesulfonyl chloride.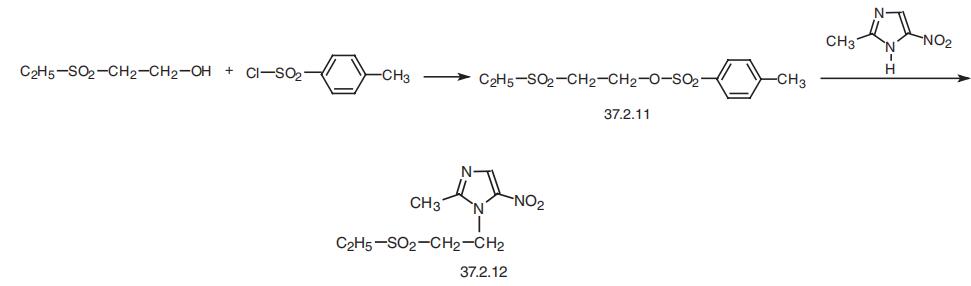
| [Veterinary Drugs and Treatments]
Little information is presently available on the use of tinidazole in
dogs, cats, or horses. It potentially could be useful for treating anaerobic
infections, particularly associated with dental infections in
small animals. Because of its antiprotozoal effects, it has been used
as an alternative for treating giardiasis in small animals, and it could
have efficacy against amebiasis, trichomoniasis or balantidiasis in
veterinary species, but documentation of efficacy is not available.
Tinidazole has a longer duration of action in dogs and cats than
does metronidazole.
In humans, oral tinidazole is FDA-approved for treating extraintestinal
and intestinal amebiasis, (Entamoeba histolytica), giardiasis
(Giardia duodenalis/lamblia), and trichomoniasis (T. vaginalis). | [Drug interactions]
Potentially hazardous interactions with other drugs
Alcohol: disulfiram-like reaction. | [Metabolism]
Tinidazole is excreted by the liver (up to 5
%) and kidneys
as unchanged drug and metabolites. An active hydroxy
metabolite has been identified. | [storage]
Store at -20°C |
|
|