| Identification | More | [Name]
4-Bromo-1-naphthylamine | [CAS]
2298-07-9 | [Synonyms]
1-AMINO-4-BROMONAPHTHALENE
4-BROMO-1-NAPHTHALENAMINE
4-BROMO-1-NAPHTHYLAMINE
1-Amino-4-bromoaphthlalene
1-Naphthalenamine, 4-bromo-
4-BROMO-1-NAPHTHALENYLAMINE
4-Bromo-1-naphtylamine
4-Bromonaphthalene-1-amine | [EINECS(EC#)]
218-944-9 | [Molecular Formula]
C10H8BrN | [MDL Number]
MFCD00004023 | [Molecular Weight]
222.08 | [MOL File]
2298-07-9.mol |
| Chemical Properties | Back Directory | [Melting point ]
102-103 °C(lit.)
| [Boiling point ]
338.4±17.0 °C(Predicted) | [density ]
1.563±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [form ]
powder to crystal | [pka]
2.93±0.10(Predicted) | [color ]
White to Amber to Dark purple | [InChI]
InChI=1S/C10H8BrN/c11-9-5-6-10(12)8-4-2-1-3-7(8)9/h1-6H,12H2 | [InChIKey]
LIUKLAQDPKYBCP-UHFFFAOYSA-N | [SMILES]
C1(N)=C2C(C=CC=C2)=C(Br)C=C1 | [CAS DataBase Reference]
2298-07-9(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi,Xn | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37/39:Wear suitable gloves and eye/face protection .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [Hazard Note ]
Irritant | [HS Code ]
29214990 | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Eye Irrit. 2
Skin Irrit. 2
STOT SE 3 |
| Hazard Information | Back Directory | [Chemical Properties]
pale purple fibrous powder | [Uses]
1-Amino-4-bromonaphthalene is a reagent used in catalytic asymmetric ring opening of meso-epoxides with aromatic amines in water. It can also be used to synthesize of 4-quinolones and quinolone heterocycles. | [Synthesis]
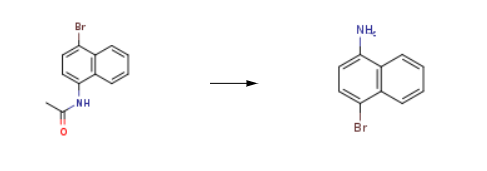
4-Bromo-1-naphthylamine is synthesised using 1-(acetylamino)-4-bromonaphthalene as a raw material by chemical reaction. The specific synthesis steps are as follows:
The 4-bromo-1-acetamido naphthalene obtained in the first step is dissolved in 100 mL of 95% ethanol, and the mass concentration of 4-bromo-1-acetylaminonaphthalene in the mixed solution is 30%, and the mass concentration is 37% added to the mixed solution. 50mL of concentrated hydrochloric acid, reacted at 80 ° C for 6h, filtered and dried to give 4-bromo-1-naphthylamine.
 | [References]
[1] Synthesis, 2004, # 17, p. 2809 - 2812
[2] Journal of the Brazilian Chemical Society, 2010, vol. 21, # 3, p. 496 - 501
[3] Journal of the Chinese Chemical Society, 2005, vol. 52, # 3, p. 559 - 562
[4] Synthetic Communications, 2005, vol. 35, # 14, p. 1947 - 1952
[5] Russian Journal of Organic Chemistry, 2007, vol. 43, # 9, p. 1282 - 1284 |
|
|