| Identification | Back Directory | [Name]
Sulfadoxine | [CAS]
2447-57-6 | [Synonyms]
fanzil
wr4873
fanasil
ro4-4393
SULFADOXIN
SULFADOXINE
sulphadoxine
SulphodoxiMe
Sulfadimoxine
Sulformetoxine
sulformethoxine
sulforthomidine
sulphormethoxine
Sulfadoxine, USP
Sulphadoxine BP98
SULPHADOXINE BP/USP
SULFADOXINE(SDM '')
Sulfadoxine (200 mg)
SulfadoxineBp98/Usp23
Sulfadoxine BP2000/USP25
Sulfadoxine Solution, 100ppm
SULFADOXINE(20FINE POWDER)/ SULFADOXINE
4-sulfanilamido-5,6-dimethoxypyrimidine
SULFADOXIN VETRANAL (4-AMINO-N-(5,6-DIME
Sulfadoxine (200 mg)F3C3360.999mg/mg(ai)
Fansidar (suldfadoxine and pyrimethamine)
n’-(5,6-dimethoxy-4-pyrimidyl)sulfanilamide
N-(5,6-Dimethoxy-4-pyrimidinyl)sulfanilamide
N'-(5,6-Dimethoxy-4-pyrimidinyl)sulfanilamide
N1-(5,6-dimethoxypyrimidin-4-yl)sulphanilamide
N1-(5,6-DiMethoxy-
4-pyriMidinyl)sulfanilaMide
6-(4-aminobenzenesulfonamido)-4,5-dimethoxypyrimidine
6-(4-aminobenzenesulfonamide)-4,5-dimethoxypyrimidine
4-amino-n-(5,6-dimethoxy-4-pyrimidinyl)-benzenesulfonamid
4-AMINO-N-(5,6-DIMETHOXY-4-PYRIMIDINYL)BENZENESULFONAMIDE
4-amino-N-(5,6-dimethoxypyrimidin-4-yl)benzenesulfonamide
4-azanyl-N-(5,6-dimethoxypyrimidin-4-yl)benzenesulfonamide | [EINECS(EC#)]
219-504-9 | [Molecular Formula]
C12H14N4O4S | [MDL Number]
MFCD00792890 | [MOL File]
2447-57-6.mol | [Molecular Weight]
310.33 |
| Chemical Properties | Back Directory | [Appearance]
White Crystalline Solid | [Melting point ]
190-194°C | [Boiling point ]
298°C (rough estimate) | [density ]
1.4006 (rough estimate) | [refractive index ]
1.6270 (estimate) | [storage temp. ]
2-8°C | [solubility ]
Soluble in ethanol & ammonium hydroxide (9:1) at 20mg/ml | [form ]
powder | [pka]
6.16±0.50(Predicted) | [color ]
white | [Water Solubility ]
209.8mg/L(temperature not stated) | [Merck ]
14,8906 | [BRN ]
625453 | [BCS Class]
3/1 | [Major Application]
clinical testing | [InChI]
1S/C12H14N4O4S/c1-19-10-11(14-7-15-12(10)20-2)16-21(17,18)9-5-3-8(13)4-6-9/h3-7H,13H2,1-2H3,(H,14,15,16) | [InChIKey]
PJSFRIWCGOHTNF-UHFFFAOYSA-N | [SMILES]
COc1ncnc(NS(=O)(=O)c2ccc(N)cc2)c1OC |
| Hazard Information | Back Directory | [Chemical Properties]
White Crystalline Solid | [Uses]
Used as an antibacterial | [Definition]
ChEBI: A sulfonamide consisting of pyrimidine having methoxy substituents at the 5- and 6-positions and a 4-aminobenzenesulfonamido group at the 4-position. In combination with the antiprotozoal pyrimethamine (CHEBI:8673) it is used as an antimalarial. | [Description]
Sulfadoxin is a sulfonamide antibiotic that inhibits dihydropteroate synthetase (DHPS), an enzyme involved in folic acid (Item No. 20515) synthesis.1 Sulfadoxin competes with 4-aminobenzoate (PABA), the native substrate of DHPS, and inhibits PABA incorporation into folic acid.2 Folate is essential for purine and pyrimidine synthesis, therefore, sulfadoxin has antiproliferative activity in non-resistant P. falciparum.2,3 Sulfadoxin inhibits growth of P. falciparum in vitro, but potency varies between non-resistant (IC50 = 4 ng/ml) and resistant strains (IC50 = 3,970 ng/ml).3 | [Originator]
Fanasil,Roche,Italy,1973 | [Manufacturing Process]
(a) α-methoxy-cyanoacetic acid methyl ester is condensed with thiourea, in
the presence of sodium methylate, to form 2-thio-4-amino-5-methoxy-6-
hydroxy-pyrimidine.
(b) The product thus obtained is methylated in a sodium methylate solution
with methyl iodide to form 2-methylthio-4-amino-5-methoxy-6-hydroxypyrimidine
of MP 203°C, from water.
(c) The latter product is methylated with phenyltrimethylammoniumtoluenesulfonate
to form 2-methylthio-4-amino-5,6-dimethoxy-pyrimidine of
MP 112° to 115°C, from 20% methanol.
(d) 0.9 gram of 2-methylthio-4-amino-5,6-dimethoxy-pyrimidine are dissolved
in 3 ml of absolute pyridine. At 0°C, 1.2 grams of pacetylaminobenzenesulfonyl
chloride are added thereto and the mixture is
shaken until all the material is dissolved. The solution is allowed to stand for
22 hours at 0°C and the pyridine eliminated in vacuo at 20°C. To the resulting
product are added 20 ml of water and 3 ml of glacial acetic acid, whereupon
the whole mixture is heated to the boil, thus causing crystallization. The crude
product obtained is dissolved in 40 ml of 2.5% soda solution, and the solution
obtained is filtered and supersaturated with gaseous carbon dioxide. There is
thus obtained 1.5 grams (85%) of 2-methylthio-4-(N4-acetyl-sulfanilamido)-
5,6-dimethoxy-pyrimidine of MP 220° to 221°C, from 50% ethanol.
(e) 1.3 grams of 2-methylthio-4-(N4-acetyl-sulfanilamido)-5,6-dimethoxypyrimidineare
dissolved in 25 ml of water and 0.4 gram of anhydrous sodium
carbonate, then refluxed for 3 ? hours in the presence of 6 to 7 grams of
Raney nickel. Then, a solution of 1 gram of sodium hydroxide in 3 ml of water
is added thereto and heating continued for another hour. The catalyst is
filtered off and the filtrate acidified to Congo red with hydrochloric acid. The
pH is then brought to 5 by means of ammonia, thus causing crystallization.
There is thus obtained 0.51 gram of 4-sulfanilamido-5,6-dimethoxy-pyrimidine
of MP 190° to 194°C, from 50% ethanol. | [Brand name]
Fanasil (Hoffmann-LaRoche-International); Fanzil (Hoffmann-LaRoche). | [Therapeutic Function]
Antibacterial | [Antimicrobial activity]
Its antibacterial activity is relatively poor. Used alone it has a
slow and uncertain effect against malaria parasites. Resistance
of malaria parasites to the combination with pyrimethamine is
common in many endemic areas. | [Pharmaceutical Applications]
An ultra-long-acting sulfonamide. It is no longer prescribed alone,
but is used in combination with pyrimethamine as the antimalarial
agent Fansidar. It is poorly soluble in water. | [Pharmacokinetics]
Oral absorption :Extensive
Cmax 500 mg oral: c. 60 mg/L after 3–4 h
Plasma half-life:c.6 days
Volume of distribution:0.13 L/kg
Plasma protein binding:94%
The extremely long half-life allows administration at weekly
intervals. The acetyl metabolite has a similarly long half-life,
but sulfadoxine is less extensively metabolized than many
other sulfonamides. | [Pharmacology]
In terms of antibacterial action, this drug is analogous to other sulfanilamides; however, it
possesses very prolonged action. Its half-life is from 120 to 200 h. Sulfadoxine is used for
infectious diseases caused by microorganisms that are sensitive to the sulfanilamide drugs,
such as infections of respiratory organs, gastric and urinary tracts; purulent infections of
various localization, osteomyelitis, sinusitis, and other infections. It is used in combination
with antimalarial drugs. Synonyms of this drug are sulfarmethoxine, fanasil, and fansidar. | [Clinical Use]
Sulfadoxine is used only in combination with pyrimethamine. | [Synthesis]
Sulfadoxine, 4,5-dimethoxy-6-sulfanilamidopyrimidine (33.1.33), is
synthesized by the standard scheme from 4-acetylaminobenzenesulfonyl chloride
and 4-amino-5,6-dimethoxypyrimidine. However, the synthesis of 4-amino-5,6-
dimethoxypyrimidine (33.1.31) is itself curious?ait is synthesized from methyl ester of
methoxyacetic acid. Interacting this with dimethyloxalate in the presence of sodium
methoxide gives the methoxy derivative (33.1.25), and the pyrolysis of this compound
gives the dimethyl ester of methoxymalonic acid (33.1.26). Reacting this with ammonia
gives the diamide of methoxymalonic acid (33.1.27). Heterocyclization of the resulting
product by a reaction with formamide in the presence of sodium ethoxide gives 4,6-
dioxy-5-methoxypyrimidine (33.1.28), which is then transformed to 4,6-dichloro-5-
methoxypyrimidine (33.1.29). The resulting 4,6-dichloro-5-methoxypyrimidine (33.1.29)
undergoes a reaction with ammonia to make 4-amino-6-chloro-5-methoxypyrimidine
(33.1.30), and the resulting compound is then reacted with sodium methoxide to make the
desired 5,6-dimethoxy-5-aminopyrimidine (33.1.31). Reacting this with 4-acety�laminobenzenesulfonyl chloride and subsequent hydrolysis of the acetyl group in
(33.1.32) gives sulfadoxine.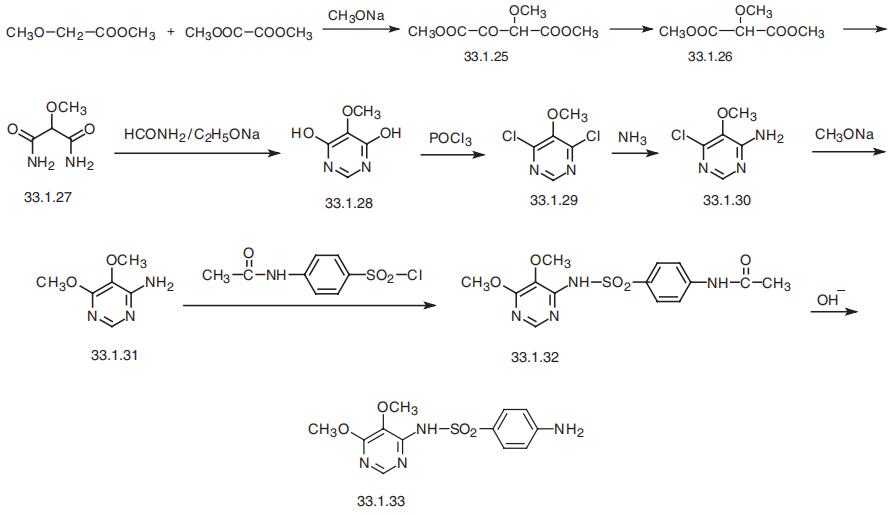
|
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
36/37/38 | [Safety Statements ]
26 | [WGK Germany ]
2 | [RTECS ]
DA9500000 | [HS Code ]
29350090 | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Aquatic Chronic 2
Skin Sens. 1 | [Toxicity]
LD50 in mice (microcrystals, mg/kg): 5200 orally, 2900 s.c., 2900 i.p. (Bhni) |
| Questions And Answer | Back Directory | [Pharmacology and mechanism of action]
The efficacy of sulphadoxine for the treatment of human malaria was first reported in 1964 [1]. Soon thereafter it was found that potentiation took place when sulphadoxine was combined with pyrimethamine for treatment of malaria and monotherapy was abandoned.
Malaria parasites synthesize their folate co-factors and cannot use dietary folic acid as the human host can. Sulphadoxine competes with para-aminobenzoic acid (PABA) for binding to the enzyme dihydropteroate synthetase in the synthesis of dihydropteroate which is an essential substance for the formation of folic acid [2]. It is active against asexual blood forms of P. falciparum but less active against other species. The action is too slow to be used alone for malaria treatment [3].
| [Indications]
Sulphadoxine is used only in combination with pyrimethamine for the treatment of falciparum malaria. It should generally not be used for malaria prophylaxis except perhaps in long-term travellers who have previously tolerated the combination.
| [Side effects]
Sulphadoxine is usually well tolerated. Vomiting, skin rashes, pruritus and haematological reactions such as haemolysis and leucopenia occur [4]. Hypersensitivity pneumonitis is reported[5, 6]. Cases of liver injury alone (hepatitis of hepatocellular, mixed hepatocellular, or aggressive type) or as part of a generalized allergic syndrome are well known[4, 6], and one case of fatal hepatic failure has also been reported [7].
Severe cutaneous adverse reactions (erythema multiforme, Stevens-Johnson syndrome, or toxic epidermal necrolysis) have been reported in persons taking prophylactic doses of sulphadoxine/pyrimethamine[6, 8]. Sulphadoxine has been incriminated as the most probable cause of these reactions. They all occurred within 7 weeks after start of prophylaxis.
The reported incidence was 1/5,000–8,000 users in USA and approximately 1/10,000 users in Sweden with fatality rates of 1/11,000–25,000 and 1/50,000, respectively. In Mozambique, when sulphadoxine was given alone in a single dose for cholera prophylaxis to 149,000 inhabitants, a total of 22 cases of Stevens-Johnson syndrome was seen with 3 deaths [9].
| [Contraindications and precautions]
The drug or its combination should not be given to patients allergic to sulphonamides. It should not be used in persons with severe blood, kidney or liver diseases.
| [Interactions]
Increased impairment of folic acid synthesis and consequent haematological adverse effects may occur if trimethoprim or its combination with sulphonamide is administered concurrently. Sulphadoxine potentiates the action of warfarin and thiopentone [10].
| [Preparations]
Sulphadoxine combined with pyrimethamine.
• Fansidar® (Roche). Tablets (sulphadoxine 500 mg+pyrimethamine 25 mg), solution for intramuscular injection (sulphadoxine 200 mg/ml+pyrimethamine 10 mg/ml).
| [References]
1. Laing ABG (1964). Antimalarial effect of sulphorthodimethoxine (Fanasil). BMJ, 2, 1439–1440.
2. The biology of malaria parasites. Technical Report Series no. 743 (1987). (Geneva: World Health Organization).
3. Chemotherapy of Malaria. Monograph series No. 27, 2nd edn, (1986), (Geneva: World Health Organization).
4. Hoigné R, Malinverni R, Sonntag R (1992). Sulfonomides, other folic acid antagonists and miscellaneous antibacterial drugs. In: Meyler’s Side Effects of Drugs, 12th edn, edited by M.N.G.Dukes (Amsterdam: Elsevier), pp. 715–722.
5. Svanbom M, Rombo L, Gustafsson L (1984). Unusual pulmonary reaction during short term prophylaxis with pyrimethamine-sulphadoxine (Fansidar). BMJ, 1, 1876.
6. Hellgren U, Rombo L, Berg B, Carlson J, Wiholm B-E (1987). Adverse reactions to sulphadoxinepyrimethamine in Swedish travellers: implications for prophylaxis. BMJ, 295, 365–366.
7. Zitelli BJ, Alexander J, Taylor S (1987). Fatal hepatic necrosis due to pyrimethamine-sulphadoxine (Fansidar). Ann Intern Med, 106, 393–395. 25. Miller KD, Lobel HO, Satriale RF, Kirutsky JN, Stern R, Campell CC (1986). Severe cutaneous reactions among American travellers using pyrimethamine-sulphadoxine (Fansidar) for malaria prophylaxis. Am J Trop Hyg, 35, 451–458.
8. Hernberg A (1985). Stevens-Johnson syndrome after mass prophylaxis with sulphadoxine for cholera in Mozambique. Lancet, 2, 1072–1073.
9. Sulfadoxine. Therapeutic Drugs, edited by Sir Colin Dollery (1991), (London: Churchill Livingstone), pp. S115–S119.
|
|
|