| Identification | Back Directory | [Name]
Metronidazole | [CAS]
443-48-1 | [Synonyms]
Cont
Nida
Nalox
Klion
Klont
Eumin
Bexon
CLONT
ARILIN
flagyl
Tricom
Fossol
Elyzol
Flagil
Flegyl
rp8823
Satric
sc10295
RP 8823
Orvagil
Mexibol
Monagyl
Monasin
Fossyol
Entizol
Efloran
Danizol
Atrivyl
Trichex
Vagilen
Vagimid
Trimeks
Zadstat
Nidazol
Trivazol
Vertisal
Wagitran
Tricocet
Trikamon
Trikojol
Trikozol
Acromona
Deflamon
MetroGel
Metrolag
Metrolyl
Flagesol
Metrotop
neo-Tric
Rathimed
SC 10295
Takimetol
Trichazol
NSC-50364
Protostat
Flagemona
Meronidal
bayer5360
Giatricol
tricowasb
Trikacide
Trichomol
Trichopal
Trichopol
NSC 69587
Trichocide
Tricowas B
Gineflavir
Anagiardil
Bayer 5360
Metro I.V.
Metronidaz
Novonidazol
Sanatrichom
Metronidazol
AKOS BB-9514
Metroniazole
DentaMet gel
METRONIDAZOLE
Tricho cordes
METRONIDAZO1E
Chont (Bayer)
Metronidazolo
Methronidazole
Cortex Albiziae
CiMetrol 500LPCI
METRONIDAZOLE BP
Tricho-gynaedron
mexibol’silanes’
component of Rtu
Metro cream & gel
AKOS BBS-00004560
METRONIDAZOLE USP
Metronidazole,99%
Metronidazole COS
METRONIDAZOLE(INN)
Metronidazole Base
METRONIDAZOLE BP98
Deflamon-Wirkstoff
TIMTEC-BB SBB001486
Atrivyl (May & Baker)
METRONIDAZOLE BIOXTRA
METRONIDAZOLE B.P.2000
Metronidazole, 99% 5GR
Metronidazole (100 mg)
component of Metro i.v.
component of Flagyl i.v.
Metronidazole|Metronidaz
METRONIDAZOLE SIGMAULTRA
METRONIDAZOLE PLAIN BP.9
METRONIDAZOLE PLAIN BP.98
trichomonacid’pharmachim’
Trichomonacid 'pharmachim'
Metronidazole CP,EP,USP,JP
Metronidazole solution
1H-Imidazole-1-ethanol,2-met
Metronidazole Base & Benzoate
METRONIDAZOL VETRANAL, 250 MG
(2-methyl-5-nitro-1-imidazolee
metronidazole Solution, 100ppm
2-METHYL-5-NITRO-1-IMIDAZOLEETHANOL
2-METHYL-5-NITRO-1-IMIDAZOLETHANOLE
2-METHYL-5-NITROIMIDAZOLE-1-ETHANOL
2-methyl-5-nitro-imidazole-1-ethano
2-Met-hyl-5-nitroimidazol-1-ylethanol
Imidazole-1-ethanol, 2-methyl-5-nitro-
2-Methyl-5-nitro-1H-imidazole-1-ethanol
1H-Imidazole-1-ethanol,2-methyl-5-nitro-
1-Hydroxyethyl-2-methyl-5-nitroimidazole
2-METHYL-5-NITROIMIDAZOLE-1-ETHANOL 99+%
2-Methyl-5-nitro-1-imidazoleethanol ,99%
2-(2-Methyl-5-nitro-1-iMidazolyl)ethanol
2-(2-methyl-5-nitroimidazol-1-yl)ethanol
1H-Imidazole-1-ethanol, 2-methyl-5-nitro-
Metronidazole (100 mg)J0C3161.000mg/mg(dr)
Metronidazole, Meets B.P. 88 Specifications
1-(beta-Oxyethyl)-2-methyl-5-nitroimidazole
1-(2-hydroxyethyl)-2-methyl-5-nitroimidazole
2-(2-Methyl-5-nitro-1H-imidazol-1-yl)ethanol
2-Methyl-1-(2-hydroxyethyl)-5-nitroimidazole
2-Methyl-3-(2-hydroxyethyl)-4-nitroimidazole
Metronidazole (base and/or unspecified salts)
1-(BETA-ETHYLOL)-2-METHYL-5-NITRO-3-AZAPYRROLE
2-(2-METHYL-5-NITRO-1H-IMIDAZOL-1-YL)ETHAN-1-OL
1-(beta-Hydroxyethyl)-2-methyl-5-nitroimidazole
1-(2-Hydroxy-1-ethyl)-2-methyl-5-nitroimidazole
Metronidazole,2-Methyl-5-nitroimidazole-1-ethanol
2-METHYL-5-NITROIMIDAZOLE-1-ETHANOL(METRONIDAZOLE)
METRONIDAZOLE 200 MG PER VIAL SIGMA*REFE RENCE STAND
2-methyl-5-nitro-1H-imidazole-1-ethanol (metronidazole)
2-Methyl-5-nitroimidazole-1-ethanol
2-(2-Methyl-5-nitro-1H-imidazol-1-yl)ethan-1-ol | [EINECS(EC#)]
207-136-1 | [Molecular Formula]
C6H9N3O3 | [MDL Number]
MFCD00009750 | [MOL File]
443-48-1.mol | [Molecular Weight]
171.15 |
| Chemical Properties | Back Directory | [Appearance]
Metronidazole is an odorless, white, yellow, or
cream-colored crystalline solid. Darkens on exposure to light.
Bitter, salty taste (do not test). | [Melting point ]
159-161 °C(lit.)
| [Boiling point ]
301.12°C (rough estimate) | [density ]
1.3994 (rough estimate) | [refractive index ]
1.5800 (estimate) | [Fp ]
9℃ | [storage temp. ]
2-8°C
| [solubility ]
acetic acid: 0.1 M, clear, faintly yellow
| [form ]
crystalline
| [pka]
pKa 2.62(H2O,t =25±0.2,Iundefined) (Uncertain) | [color ]
white to light yellow | [Stability:]
Stable. Incompatible with strong oxidizing agents. | [Water Solubility ]
<0.1 g/100 mL at 20 ºC | [Merck ]
6157 | [BRN ]
611683 | [BCS Class]
1,3 | [Major Application]
clinical testing | [InChI]
1S/C6H9N3O3/c1-5-7-4-6(9(11)12)8(5)2-3-10/h4,10H,2-3H2,1H3 | [Contact allergens]
Metronidazole is a nitro-6-imidazole compound with antiprotozoal and antibacterial properties. Topical exposure may induce allergic contact dermatitis. Sensitization is mainly observed with the treatment of rosacea and rarely occurs from handling of table돐㢦돰㢦됐㢦뺀㢦랠㢦눐㢦념㢦쀘㢦쀸㢦쁘㢦삘㢦샘㢦샸㢦세㢦션㢦섘㢦셸㢦솘㢦숈㢦숨㢦쉈㢦쉰㢦슐㢦슰㢦싐㢦싰㢦짨㢦패㢦쌐㢦 | [InChIKey]
VAOCPAMSLUNLGC-UHFFFAOYSA-N | [SMILES]
CC1=NC=C([N+]([O-])=O)N1CCO | [LogP]
-0.02 | [IARC]
2B (Vol. 13, Sup 7) 1987 | [NIST Chemistry Reference]
Metronidazole(443-48-1) | [EPA Substance Registry System]
1H-Imidazole-1-ethanol, 2-methyl-5-nitro-(443-48-1) |
| Hazard Information | Back Directory | [Chemical Properties]
white to slightly yellow crystalline powder | [Uses]
Used as an antibacterial in the treatment of rosacea. Antiprotozoal (trichomonas). A potential human carcinogen. | [Definition]
ChEBI: A member of the class of imidazoles substituted at C-1, -2 and -5 with 2-hydroxyethyl, nitro and methyl groups respectively. It has activity against anaerobic bacteria and protozoa, and has a radiosensitising effect on hypoxic tumour cells. It may be given
by mouth in tablets, or as the benzoate in an oral suspension. The hydrochloride salt can be used in intravenous infusions. Metronidazole is a prodrug and is selective for anaerobic bacteria due to their ability to intracellularly reduce the nitro group of
metronidazole to give nitroso-containing intermediates. These can covalently bind to DNA, disrupting its helical structure, inducing DNA strand breaks and inhibiting bacterial nucleic acid synthesis, ultimately resulting in bacterial cell death. | [General Description]
White to pale-yellow crystalline powder with a slight odor. Bitter and saline taste. pH (saturated aqueous solution) about 6.5. | [Air & Water Reactions]
Insoluble in water. | [Reactivity Profile]
Metronidazole darkens on exposure to light. Metronidazole is incompatible with strong oxidizing agents. . | [Fire Hazard]
Flash point data for Metronidazole are not available; however, Metronidazole is probably combustible. | [Potential Exposure]
Metronidazole is an orally administered
drug for the treatment of infections due to entamoeba
histolytica; trichomonas vaginalis; giardia lamblia, and has
also been used for treating Vincent’s infection. It can be
used as a trichomonacide in veterinary medicine. One firm
has petitioned EPA to use metronidazole as a disinfectant
for cooling tower water. | [First aid]
Skin Contact: Flood all areas of body that
have contacted the substance with water. Don’t wait to remove contaminated clothing; do it under the water
stream. Use soap to help assure removal. Isolate contaminated
clothing when removed to prevent contact by others.
Eye Contact: Remove any contact lenses at once. Flush
eyes well with copious quantities of water or normal saline
for at least 20 30 minutes. Seek medical attention.
Inhalation: Leave area immediately; breathe fresh air.
Proper respiratory protection must be supplied to any
rescuers. If coughing, difficult breathing or any other
symptoms develop, seek medical attention at once, even
if symptoms develop many hours after exposure. Ingestion:
If convulsions are not present, give a glass or two of water or
milk to dilute the substance. Assure that the person’s airway
is unobstructed and contact a hospital or poison center
immediately for advice on whether or not to induce vomiting. | [Shipping]
UN3249 Medicine, solid, toxic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials. | [Incompatibilities]
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions.
Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides. | [Description]
Metronidazole is a nitroimidazole antibiotic first isolated in the
1950s. Many nitroimidazoles were being studied at the time,
as the class was found to have trichomonacidal properties.
Metronidazole was of particular interest due to its high activity
against Trichomonas vaginalis and Entamoeba histolytica both
in vitro and in vivo as well as clinical activity against many
anaerobic pathogens including Gram-positive and Gramnegative
bacteria and Giardia lamblia. Metronidazole is often
used clinically for intra-abdominal infections and is the firstline
treatment for Clostridium difficile–associated diarrhea. | [Waste Disposal]
Dispose of contents and
container to an approved waste disposal plant. All federal,
state, and local environmental regulations must be observed.
It is inappropriate and possibly dangerous to the environment
to dispose of expired or waste drugs and pharmaceuticals by
flushing them down the toilet or discarding them to the trash.
Household quantities of expired or waste pharmaceuticals
may be mixed with wet cat litter or coffee grounds, doublebagged
in plastic, discard in trash. Larger quantities shall
carefully take into consideration applicable DEA, EPA, and
FDA regulations. If possible return the pharmaceutical to the
manufacturer for proper disposal being careful to properly
label and securely package the material. Alternatively, the
waste pharmaceutical shall be labeled, securely packaged,
and transported by a state licensed medical waste contractor
to dispose by burial in a licensed hazardous or toxic waste
landfill or incinerator. | [Originator]
Flagyl,Specia,France,1960 | [Manufacturing Process]
2-Methyl-4(or 5)-nitroimidazole (127 g) is heated with ethylene chlorohydrin
(795 g) for 18 hours at 128° to 130°C and the chlorohydrin (660 g) is then
distilled under reduced pressure (30mm Hg). The residue is treated with water
(300 cc) and filtered, and the filtrate is made alkaline by the addition of
sodium hydroxide solution (d = 1.33, 100 cc). It is then extracted with
chloroform (1,000 cc) and, after evaporation of the chloroform in vacuo, there
is obtained a pasty mass (77 g) which is recrystallized from ethyl acetate (450
cc) in the presence of animal charcoal. There is thus obtained 1-(2-
hydroxyethyl)-2-methyl-5-nitroimidazole (24 g) as a creamy white crystalline
powder melting at 158° to 160°C. | [Brand name]
Flagyl (Searle); Metrogel (Galderma); Metrogel (3M Pharmaceuticals); Noritate(Sanofi Aventis); Vandazole (Teva). | [Therapeutic Function]
Antiprotozoal | [Antimicrobial activity]
It is a potent inhibitor of obligate anaerobic bacteria and protozoa, but not of any organism that is aerobic or incapable of anaerobic metabolism. Susceptible protozoa include T. vaginalis, G. lamblia, E. histolytica, Balantidium coli and Blastocystis hominis, which are inhibited by concentrations of 0.2–0.25 mg/L. Clostridium spp. (including C. difficile) are inhibited at concentrations of 0.5–8 mg/L. It is also active against the microaerophilic H. pylori (MIC for susceptible strains <8 mg/L). The 2-methoxy metabolite of metronidazole is more active (MIC about 0.3 mg/L), but the acid metabolite shows less activity than the parent drug (MIC about 3 mg/L). G. vaginalis shows similar susceptibility (MIC 1–8 mg/L); the methoxy metabolite is more active (MIC 0.02–2 mg/L). | [Antimicrobial activity]
Metronidazole inhibits E. histolytica, G. lamblia, T. vaginalis,
Blastocystis hominis, B. coli, and the helminth
Dracunculus medinensis. It is also bactericidal for obligate
anaerobic gram-positive and gram-negative bacteria
except Actinomyces spp. It is not active against aerobes
or facultative anaerobes. Drug resistance is
infrequent; the mechanism of resistance is not understood.
Tinidazole, a 5-nitroimidazole closely related to
metronidazole, is effective against vaginal trichomoniasis
resistant to metronidazole. | [Acquired resistance]
Although resistance in Bacteroides spp. and T. vaginalis is well documented, it is uncommon. Resistance occurs more frequently in H. pylori and failure of treatment with triple drug regimens may be associated with resistance to the metronidazole component. | [Pharmaceutical Applications]
A 5-nitroimidazole available for oral administration or as a suppository; also formulated as the hydrochloride for intravenous use, and as the benzoate in an oral suspension and a dental gel. Aqueous solubility: 10 g/L at 20°C. Soluble in dilute acids. It is photolabile and preparations should be protected from light. Metronidazole hydrochloride has a low pH (0.5–2.0) when reconstituted, and reacts with aluminum in equipment, including needles, to produce a reddish-brown discoloration. It is incompatible with several agents and other drugs should not be added to intravenous solutions. | [Biochem/physiol Actions]
Metronidazole is a prodrug and is selective for anaerobic bacteria due to their ability to intracellularly reduce metronidazole to its active form. Reduced metronidazole covalently binds to DNA which disrupts its helical structure, induces DNA strand breaks and inhibits bacterial nucleic acid synthesis. Bacterial cell death results. | [Mechanism of action]
Despite the availability of metronidazole since the late 1950s, the mechanism of action of the drug
is still unknown. It generally is agreed that metronidazole is a pro-drug and that anaerobic
organisms reduce the nitro group in metronidazole to a hydroxylamine, as shown in Figure 39.2,
during which a reactive derivative or reactive species are produced that cause destructive effects
on cell components (i.e., DNA, proteins, and membranes). Specifically, DoCampo has reported
that nitroaryl compounds (nitroimidazoles, metronidazole; nitrofurans, nifurtomox) are reduced to
nitro radical anions, which in turn react with oxygen to regenerate the nitroaryl and the superoxide
radical anion. Further reduction of superoxide radical anion leads to hydrogen peroxide
and homolytic cleavage of the latter leads to hydroxyl radical formation. Superoxide radical anion,
hydrogen peroxide, and hydroxyl radicals are referred to as reactive oxygen species (ROS) and
are the reactive substances that are implicated in damage to critical cellular components of the
parasite. | [Pharmacokinetics]
Oral absorption :>90%
Cmax 400 mg oral :c. 10 mg/L after 3–5 h
Plasma half-life: 6–11 h
Volume of distribution:0.6–1.1 L/kg
Plasma protein binding:<20%
absorption
Peak plasma concentrations after oral administration are proportional to the dose. Plasma levels are usually lower in men because of weight differences. In patients treated intravenously with a loading dose of 15 mg/kg followed by 7.5 mg/kg every
6 h, peak steady state plasma concentrations averaged 25 mg/L with minimum trough concentrations averaging 18 mg/L.
The bioavailability of metronidazole in rectal suppositories is around 60%. Effective blood concentrations occur 5–12 h after the first suppository and are maintained by an 8 h regimen.
There are conflicting data on the effects of age on absorption. One study, which did not distinguish between metronidazole and its metabolites, indicated that the area under the curve (AUC) for plasma was almost doubled in the elderly. However, the general consensus is that there is no requirement for a decreased dosage for the elderly, unless there is significant renal impairment.
Distribution
It is widely distributed in body tissues after oral or intravenous administration.It appears about 90 min after an oral dose in brain tissue, cerebrospinal fluid (CSF), saliva and breast milk in concentrations similar to those found in plasma: and in :vaginal secretions, pleural and prostatic fluid at levels about 40% of those of the plasma. In patients receiving 500 mg every 12 h or 1 g every 6 h, CSF levels of up to 2 and 8 mg/L, respectively, have been found. Bactericidal concentrations of metronidazole are achieved in pus from hepatic abscesses. Concentrations in placenta and fetal tissue are related to the corresponding maternal plasma levels: concentrations of 3.5 mg/kg (placenta) and 9 mg/kg (fetus) when the plasma concentration was 13.5 mg/L.
Metabolism
It is metabolized in the liver to a glucuronide conjugate and to acid and hydroxy derivatives. The acid metabolite, produced by oxidation of the N-1 ethanol side-chain, is microbiologically inactive and appears in the urine because of its high water solubility. The hydroxy derivative, which is as active as the parent drug against G. vaginalis, is formed by oxidation of the methyl group on C-2 of the imidazole ring, first to the hydroxymethyl derivative and subsequently to the carboxylic acid. Hydroxymetronidazole has a half-life of 10–13 h. Both metronidazole itself and the hydroxymethyl metabolite can form sulfate or glucuronide conjugates: the acid metabolite may be excreted as the glycine conjugate. Traces of metabolites derived from reduction of the nitro group are found in urine and are assumed to be formed by the intestinal flora.
excretion
About 60–80% of the dose appears in the urine and 6–15% in the feces. The hydroxy and acid metabolites are also excreted in the urine. Glucuronide conjugates account for approximately 20% of the total. Renal clearance is approximately 10 mL/min per 1.73 m2. Decreased renal function does not alter the single-dose kinetics and dose adjustment is not normally required in patients with renal impairment. However, the hydroxy metabolite may accumulate in patients with end-stage disease and dose reduction may be necessary. Elimination is prolonged in patients with impaired liver function necessitating dose reduction. Hemodialysis increases the clearance of metronidazole, shortening the half-life to 2–3 h.
Newborn infants possess a decreased capacity to eliminate metronidazole. In one study, the elimination half-life measured during the first 3 days of life was inversely related to gestational age. In premature newborns and infants whose gestational ages were between 28 and 40 weeks, the corresponding half-life elimination rates ranged from 10.9 to 22.5 h. | [Pharmacology]
Absorption from the intestinal tract is usually good.
Food delays but does not reduce absorption.The drug is
distributed in body fluids and has a half-life of about 8
hours. High levels are found in plasma and cerebrospinal
fluid (CSF). Less than 20% binds to plasma
proteins. Metronidazole is metabolized by oxidation
and glucuronide formation in the liver and is primarily
excreted by the kidneys, although small amounts can be
found in saliva and breast milk. Dose reduction is generally
unnecessary in renal failure. | [Clinical Use]
It is also used in acne rosacea, balantidiasis and Guinea worm infection. T. vaginalis infections resistant to the usual dosage require special treatment. | [Clinical Use]
Metronidazole is the most effective agent available for
the treatment of individuals with all forms of amebiasis,
with perhaps the exception of the person who is asymptomatic
but continues to excrete cysts. That situation
calls for an effective intraluminal amebicide, such as
diloxanide furoate, paromomycin sulfate, or diiodohydroxyquin.
Metronidazole is active against intestinal
and extraintestinal cysts and trophozoites.
Although quinacrine hydrochloride has been used
for the treatment of giardiasis, many physicians prefer
metronidazole. Furazolidone is an alternate choice.
Metronidazole is the drug of choice in Europe for
anaerobic bacterial infections; concern about possible
carcinogenicity has led to some caution in its use in the
United States.Recently it has been found to be effective
in treating D. medinensis (Guinea worm) infections and
Helicobacter pylori. | [Synthesis]
Metronidazole, 2-methyl-5-nitroimidazol-1-ethanol (37.2.10), is made by
nitrating 2-methylimidazole to make 2-methyl-5-nitroimidazole (37.2.9), which is then
reacted with 2-chloroethanol or ethylenoxide, which is easily transformed to the desired
metronidazole.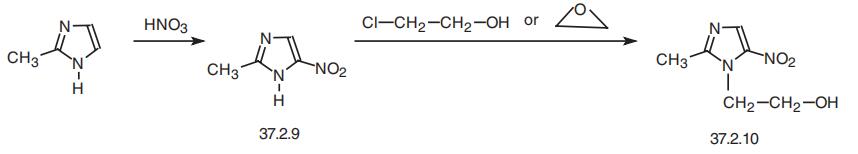
| [Veterinary Drugs and Treatments]
Although there are no veterinary-approved metronidazole products,
the drug has been used extensively in the treatment of Giardia in
both dogs and cats. It is also used clinically in small animals for the
treatment of other parasites (Trichomonas and Balantidium coli) as
well as treating
both enteric and systemic anaerobic infections.
In horses, metronidazole has been used clinically for the treatment
of anaerobic infections. | [Drug interactions]
Potentially hazardous interactions with other drugs
Alcohol: disulfiram-like reaction.
Anticoagulants: effects of coumarins enhanced.
Antiepileptics: metabolism of phenytoin inhibited;
concentration reduced by phenobarbital.
Busulfan: concentration of busulfan increased - risk
of toxicity.
Ciclosporin: raised blood level of ciclosporin.
Cytotoxics: busulfan concentration increased;
metabolism of fluorouracil inhibited. | [Carcinogenicity]
Metronidazole is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals. | [Environmental Fate]
Due to metronidazole’s use as a pesticide, it may have been
directly released into the environment. It lacks an adequate
chromophore for absorbing light and undergoing photolytic
degradation. In addition, in vitro assays demonstrated the
compound’s robust stability in the atmosphere or aqueous
environments. Metronidazole exhibited a soil half-life between
10 and 27 days. | [Metabolism]
Metronidazole is available in a variety of dosage forms, including IV, oral, rectal, and vaginal
suppositories. The bioavailability of metronidazole is nearly 100% when administered orally but is
significantly less when administered via the rectal route (67–82%) or the vaginal route (19–56%).
The drug is not bound to plasma protein. Distribution of the drug is fairly uniform through out the
body, including mother's milk.
Liver metabolism of metronidazole leads to two major metabolites: hydroxylation of the 2-methyl
group to 2-hydroxymethylmetronidazole (HM), and oxidation to metronidazole acetic acid. Both
compounds possess biological activity. Additionally, HM is found in the urine as glucuronide and
sulfate conjugates. In addition, a small amount of metronidazole is oxidized to acetamide, a known
carcinogen in rats but not in humans. | [storage]
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in a refrigerator. A regulated, marked areashould be established where this chemical is handled, used,or stored in compliance with OSHA Standard 1910.1045. | [Toxicity evaluation]
Metronidazole is a prodrug that requires reductive activation
of the nitro group by susceptible organisms. The reduction
causes nitro radical formation and destruction of the organism’s
DNA. The mechanism of neurotoxicity is thought to be
due to axonal degeneration. Metronidazole has been shown to
bind neuronal RNA in rodent models, thus inhibiting protein
synthesis and causing degeneration. Metronidazole is also
capable of producing a disulfiram-type reaction with ethanol
ingestion. This reaction is hypothesized to occur due to
metronidazole inhibition of aldehyde dehydrogenase. |
| Questions And Answer | Back Directory | [Antibacterial Spectrum]
In addition to being used for anti-trichomoniasis and anti-ameba, in recent years, metronidazole has been widely used in anti-anaerobic infection. The nitro group of this product is reduced to amino group in an anaerobic environment and shows the effect of anti-anaerobic bacteria, but it is ineffective against aerobic bacteria or facultative aerobic bacteria. It has good antibacterial effect on the following anaerobic bacteria: ① Bacteroides, including Bacteroides fragilis; ② Clostridium; ③ Clostridium, including Tetanus; ④ Partial Eubacterium; ⑤ Peptococcus and Digestive Streptococcus etc. | [Brand Name(s)]
Flagyl and generic
| [Indications and Usage]
Metronidazole is a nitroimidazole antibiotic, also known as metronidazol and novonidazol. It was initially used to treat vaginal trichomaniasis, with very significant clinical effects. It is broadly used to prevent and treat oral anaerobic infections. In hospitals, it has been used frequently to prevent and treat respiratory, gastrointestinal, peritoneal, pelvic, skin, soft tissue, joint, and brain infections, cardiomyitis, and septicemia caused by anaerobic bacteria. The effectiveness of Metronidazole towards treating body tissue and intestinal amoebiasis is significant, and it the preferred drug to treat parasitosis.
| [Mechanisms of Action]
Metronidazole kills anaerobic microorganisms, and its metabolites in the body during reduction also inhibit them by inhibiting DNA synthesis, thus interfering with bacterial growth and propagation, eventually killing them. Anaerobic bacteria affected include: Bacteroides fragilis, Fusobacterium (so named because of its sharp fusiform shape at both ends,) Clostridium tetani, Peptostreptococcus, and Giardia lamblia. Its mechanism of action in the treatment of parasites is to disrupt protozoans’ nitrogen chains by inhibiting their redox reactions. In vitro experiments have shown that at concentrations of 1-2 mg/L, morphological changes occurred in dissolved amoeba starting at 6-20 hours, killing them all within 24 hours. At a concentration of 0.2 mg/L, dissolved bacteria were killed within 72 hours.
| [Warnings and Precautions]
Interactions with nitroimidazole antibiotics, ethanol, and nicotine interfere with the oxidation of ethanol and can cause disulfiram reactions, causing symptoms like faster heart rate and decreased blood pressure, so patients should avoid contact with alcohol and smoke less during treatment in order to prevent the occurrence of adverse reactions.
| [Methods of production]
It is synthetized by 2-methyl-5-nitro imidazole (see 25010) and ethylene oxide addition. 2-methyl-5-nitro imidazole dissolved in formic acid and at 30-40℃ successive adding epoxy ethane, and sulfuric acid in the middle of adding feeding. and reaction for 1 h, after that. Decompression to recycle formic acid, water solution is cooled to 10 ℃, filter. The filtrate with sodium hydroxide solution to adjust pH = 10. Set aside to cool, filtering, washing to nearly alterations into neutral, recrystallization in water. Activated carbon decolorization to get metronidazole.
| [Pharmacology and mechanism of action]
Metronidazole is a 5-nitroimidazole derivative which was originally introduced against Trichomonas vaginalis in 1960. Soon it was shown to possess a broad spectrum of activity against other protozoal infections such as amoebiasis and giardiasis, and more recently against infections due to anaerobic bacteria [1]. The mechanism of action of metronidazole is not well understood. In the parasite, the 5-nitro group of the drug undergoes reductive transformation to a cytotoxic intermediate which binds to the helical structure of the DNA leading to strand breakage and eventual cell death [2].
| [Indications]
Against infections caused by Trichomonas vaginalis, Entamoeba histolytica (acute intestinal type and liver abscesses), Giardia lamblia and Dracunculus medinensis. During treatment of trichomoniasis it is wise to treat the male partner as well. In amoebiasis, a luminal amoebicide is added to eliminate surviving organisms in the colon. Metronidazole is also used for the treatment of infections due to anaerobic bacteria.
| [Side effects]
Side effects with doses used to treat protozoal infections are usually mild, reversible and self-limiting and may affect 4% to 5% of treated patients. The most common are gastrointestinal disturbances (nausea, vomiting, epigastric pain, metallic taste, furring of the tongue), intolerance to alcohol (disulfiram-like effect) and central nervous system effects (headache, dizziness and sleepiness) [3]. Other side effects reported include urticaria, darkening of the urine with a reddish-brown discoloration and transient neutropenia [4]. During prolonged high doses, the drug may cause severe neurotoxic side effects such as peripheral neuropathy, paraesthesia and epileptiform seizures [3,4]. Few case reports of bone marrow depression [5], gynecomastia [6] and acute pancreatitis [7] have been reported.
Although metronidazole is mutagenic in bacteria and carcinogenic in rodents, no association with human cancer has been proven .
| [Contraindications and precautions]
Dosage reductions should be made in patients with severe hepatic failure. Because of its potential neurotoxicity and neutropenia the drug should be given with caution to patients with diseases of the CNS or with a history of blood dyscrasia. Patients should be warned of a disulfiram-like reaction if the drug is taken together with alcohol. Metronidazole should be used with extra caution in patients being treated with warfarin (see interactions).
| [Interactions]
Metronidazole is a weak inhibitor of alcohol dehydrogenase. Simultaneous administration of metronidazole and disulfiram has been reported to cause an acute psychosis or mental confusion. This effect was observed in 6 of 29 chronic alcoholic men given both drugs, but in none of those given placebo plus disulfiram [8]. Metronidazole inhibits the ring oxidation of S (+) warfarin and significant bleeding can occur if the two drugs are taken together [9]. Significant increase of hepatic clearance of metronidazole has been reported when the drug was taken together with phenobarbital [10, 11] or prednisone [11].
| [Preparations]
Many preparations are available apart from those mentioned below. Available as metronidazole
• Elyzol® (Dumex). Solution for infusion 5 mg/ml. Tablets 250 mg, 500 mg. Suppositories 500 mg, 1000 mg.
• Flagyl® (Rhône-Poulenc Rorer). Solution for infusion 5 mg/ml. Tablets 200 mg, 400 mg. Suppositories 500 mg, 1000 mg.
• Servizol® (Servipharm). Tablets 200 mg, 250 mg.
Available as metronidazole benzoate: 10 mg metronidazole benzoate is equivalent to 6.2 mg metronidazole.
• Elyzol (Dumex)® Oral solution 25 mg metronidazole base/ml.
• Flagyl® (Rhône-Poulenc Rorer). Oral solution 40 mg metronidazole base/ml.
| [References]
1. Scully BE (1988). Metronidazole. Med Clin North Amer, 72, 613–621.
2. Muller M (1983). Mode of action of metronidazole on anaerobic bacteria and protozoa. Surgery, 93, 165–171.
3. Lau AH, Lam NP, Piscitelli SS (1992). Clinical pharmacokinetics of metronidazole and other nitroimidazole anti-infectives. Clin Pharmacokinet, 23, 328–364.
4. Roe FJC (1985). Safety of nitroimidazoles. Scand J Infect Dis, 46, 72–81.
5. Heisterberg L, Branebjerg PE (1983). Blood and milk concentrations of metronidazole in mothers and infants. J Perinat Med, 11, 114–120.
6. Fagan TC, Johnson DG, Grosso DS (1985). Metronidazole-induced gynecomastia. J Am Med Ass, 254, 3217.
7. Poltkin BH, Cohen I, Tsang T, Cullinane T (1985). Metronidazole-induced pancreatitis. Ann Intern Med, 103, 891–892.
8. Rothstein E, Clancy DD (1969). Toxicity of disulfiram combined with metronidazole. N Engl J Med, 280, 1006–1007.
9. O’Reilly RA (1976). The stereoselective interaction of warfarin and metronidazole in man. N Engl J Med, 295, 354–357.
10. Gupte S (1983). Phenobarbital and metabolism of metronidazole. N Engl J Med, 308, 529. 11. Eradiri D, Jamali R, Thomson ABR (1988). Interaction of metronidazole with phenobarbital, cimetidine, prednisone, and sulphasalzine in Crohn’s disease. Biopharmaceut Drug Disp, 9, 219– 227.
|
| Safety Data | Back Directory | [Hazard Codes ]
Xn,T,F | [Risk Statements ]
40-46-45-39/23/24/25-23/24/25-11 | [Safety Statements ]
36/37-45-53-16-7 | [WGK Germany ]
3
| [RTECS ]
NI5600000
| [F ]
8 | [HS Code ]
29332990 | [Storage Class]
6.1C - Combustible acute toxic Cat.3
toxic compounds or compounds which causing chronic effects | [Hazard Classifications]
Carc. 1B
Muta. 1B
STOT RE 2 | [Safety Profile]
Confirmed carcinogen
with experimental carcinogenic,
neoplastigenic, tumorigenic, and teratogenic
data. Moderately toxic by ingestion,
intraperitoneal, and subcutaneous routes.
Human systemic effects by ingestion:
paresthesia, nerve or sheath structural
changes, eye changes, tremors, fever,
jaundice and other liver changes, hearingacuity
changes, somnolence, and ataxia.
Experimental reproductive effects. Human
mutation data reported. When heated to
decomposition it emits toxic fumes of NOx. | [Hazardous Substances Data]
443-48-1(Hazardous Substances Data) |
|
|