| Identification | More | [Name]
Olivetol | [CAS]
500-66-3 | [Synonyms]
3,5-dihydroxyamylbenzene
5-AMYL RESORCINOL
5-N-PENTYLRESORCINOL
5-PENTYL 1,3-BENZENEDIOL
5-PENTYLRESORCINOL
AURORA KA-7378
OLIVETOL
5-n-amylresorcinol
5-pentyl-3-benzenediol
5-pentyl-resorcino
n-Amylresolcinol
Oilvetol
Pentyl-3,5-dihydroxybenzene
Olivetol(3,5-Dihydroxyamylbenzene)
1,3-Benzenediol, 5-pentyl-
1,3-dihydroxy-5-pentylbenzene
Resorcinol, 5-pentyl.
Olivetol, GC 95%
5-Pentylresorcinol( Olivetol)
5-pentylbenzene-1,3-diol | [EINECS(EC#)]
207-908-8 | [Molecular Formula]
C11H16O2 | [MDL Number]
MFCD00002293 | [Molecular Weight]
180.24 | [MOL File]
500-66-3.mol |
| Chemical Properties | Back Directory | [Appearance]
light purple to brown crystalline mass | [Melting point ]
46-48 °C(lit.)
| [Boiling point ]
164 °C | [density ]
1.068±0.06 g/cm3(Predicted) | [Fp ]
>230 °F
| [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
9.59±0.10(Predicted) | [color ]
Colourless to Beige Oil to Low-Melting | [Stability:]
Light Sensitive | [InChI]
InChI=1S/C11H16O2/c1-2-3-4-5-9-6-10(12)8-11(13)7-9/h6-8,12-13H,2-5H2,1H3 | [InChIKey]
IRMPFYJSHJGOPE-UHFFFAOYSA-N | [SMILES]
C1(O)=CC(CCCCC)=CC(O)=C1 | [Uses]
Olivetol was used as a template molecule in the synthesis of molecularly imprinted polymer (MIP). It was also used as an inhibitor of (S)-mephenytoin 4'-hydroxylase activity of recombinant CYP2C19. | [CAS DataBase Reference]
500-66-3(CAS DataBase Reference) | [NIST Chemistry Reference]
1,3-Benzenediol, 5-pentyl-(500-66-3) | [EPA Substance Registry System]
500-66-3(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/39:Wear suitable protective clothing and eye/face protection . | [WGK Germany ]
3
| [RTECS ]
VH2880000
| [HS Code ]
2907290090 | [Storage Class]
6.1C - Combustible acute toxic Cat.3
toxic compounds or compounds which causing chronic effects | [Hazard Classifications]
Acute Tox. 4 Oral
Aquatic Acute 1
Aquatic Chronic 3
Eye Dam. 1
Skin Irrit. 2
Skin Sens. 1B
STOT SE 1 Oral
STOT SE 2 Oral |
| Hazard Information | Back Directory | [General Description]
Off-white crystals or olive to light purple waxy solid. Forms monohydrate (melting point: 102-106°F). | [Reactivity Profile]
OLIVETOL(500-66-3) is incompatible with acid chlorides, acid anhydrides and oxidizing agents. | [Air & Water Reactions]
Sensitive to air. Insoluble in water. | [Fire Hazard]
This chemical is probably combustible. | [Chemical Properties]
light purple to brown crystalline mass | [Chemical Properties]
OLIVETOL is an off-white crystals or olive to light purple waxy solid. Forms monohydrate (melting point: 102-106°F). Olivetol is a member of the class of resorcinols that is resorcinol in which the hydrogen at position 5 is replaced by a pentyl group. It has a role as a lichen metabolite. | [Physical properties]
light purple to brown crystalline mass. | [Application]
Olivetol is a precursor in various syntheses of tetrahydrocannabinol. | [Definition]
ChEBI: A member of the class of resorcinols that is resorcinol in which the hydrogen at position 5 is replaced by a pentyl group. | [Preparation]
olivetol can also be formed through hydrolysis of intermediate polyketide CoAs or spontaneous cyclization.
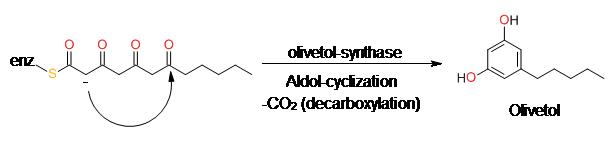 | [Synthesis Reference(s)]
The Journal of Organic Chemistry, 42, p. 3456, 1977 DOI: 10.1021/jo00441a036 | [Biological Activity]
Olivetol (5-Pentylresorcinol, 5-n-Amylresorcinol) is a naturally occurring organic compound being a precursor in various syntheses of tetrahydrocannabinol. It acts as a competitive inhibitor of the cannabinoid receptors CB1 and CB2.? | [Safety]
Olivetol is still a very new product. It has not been researched nearly enough to prove it's safe to use. So far, no one has reported any adverse side effects as far as we know. Until more information is available, carefully control your THC intake instead of relying on an untested gelcap. | [storage]
Store at 2-8°C, stored under nitrogen | [Mode of action]
When you look at olivetol's molecular structure, it'll look very familiar. It's as if someone took a THC molecule and sliced it in two. Olivetol, like THC, works by binding with the CB1 receptors that exist all over your body and brain. But, olivetol is thought to be smaller and stickier than THC, so that helps it reduce a raging high in two ways. First, it slips into any open receptors before THC can get there to block them—so you won't get any higher. Second, it bumps into THC molecules that are already lodged in a CB1 receptor to knock them loose and take their place. That's how it brings you down.
According to numerous personal accounts, olivetol works, but we don't know exactly how it does so. More research and investigation is needed, but in theory it's very similar to how Narcan acts to reverse opiate overdoses. |
|
|