| Identification | More | [Name]
1,3,5(10)-Estratrien-3-ol-17-one | [CAS]
53-16-7 | [Synonyms]
1,3,5(10)-ESTRATRIEN-3-OL-17-ONE
1,3,5[10]-ESTRATRIENE-3-OL-17-ONE
1,3,5-ESTRATRIEN-3-OL-17-ONE
3beta-hydroxyestra-1,3,5(10)-trien-17-one
3-HYDROXY-1,3,5[10]-ESTRATRIEN-17-ONE
3-HYDROXY-ESTRA-1,3,5(10)-TRIEN-17-ONE
beta-estrone
DELTA1,3,5(10)-ESTRATRIEN-3-OL-17-ONE
DESTRONE
E 1
ESTROL
ESTRONE
FOLLICULIN
KESTRONE
OESTRIN
OESTRONE
OESTRONE (E1)
(8R,13S)-3-Hydroxy-13-methyl-6,7,8,9,11,12,13,14,15,16-decahydro-cyclopenta[a]phenanthren-17-one
1,3,5(10)-Oestratrien-3-ol-17-one
1,3,5-Oestratrien-3-ol-17-one | [EINECS(EC#)]
200-164-5 | [Molecular Formula]
C18H22O2 | [MDL Number]
MFCD00003620 | [Molecular Weight]
270.37 | [MOL File]
53-16-7.mol |
| Chemical Properties | Back Directory | [Appearance]
Estrone is an odorless white crystalline powder. | [Melting point ]
258-260 °C(lit.)
| [alpha ]
158 º (c=1, dioxane) | [Boiling point ]
353.48°C (rough estimate) | [density ]
1.2360 | [refractive index ]
165 ° (C=1, Dioxane) | [Fp ]
9℃ | [storage temp. ]
room temp | [solubility ]
Chloroform (Slightly), Dioxane (Slightly), Ethanol (Slightly), Methanol (Slightly) | [form ]
Crystalline Powder or Crystals | [pka]
pKa 10.77±0.02(H2O)(Approximate) | [color ]
White to almost white | [Water Solubility ]
0.03 g/L | [Usage]
A metabolite of 17-Estradiol | [Merck ]
3708 | [BRN ]
1915077 | [Major Application]
pharmaceutical
pharmaceutical small molecule | [InChI]
1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1 | [InChIKey]
DNXHEGUUPJUMQT-CBZIJGRNSA-N | [SMILES]
C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CCC2=O | [CAS DataBase Reference]
53-16-7(CAS DataBase Reference) | [NIST Chemistry Reference]
3-Hydroxyestra-1,3,5(10)-trien-17-one(53-16-7) | [EPA Substance Registry System]
53-16-7(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
R45:May cause cancer.
R60:May impair fertility.
R61:May cause harm to the unborn child. | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN1230 - class 3 - PG 2 - Methanol, solution | [WGK Germany ]
3
| [RTECS ]
KG8575000
| [TSCA ]
TSCA listed | [HS Code ]
29335995 | [Storage Class]
6.1D - Non-combustible acute toxic Cat.3
toxic hazardous materials or hazardous materials causing chronic effects | [Hazard Classifications]
Carc. 2
Lact.
Repr. 1A | [Safety Profile]
Confirmed carcinogen
with experimental carcinogenic,
neoplastigenic, tumorigenic, and teratogenic
data. A poison by intraperitoneal and
subcutaneous routes. Human reproductive
effects by implantation: spermatogenesis
and impotence. Mutation data reported. A
steroid drug for the treatment of menopause
and ovariectomy symptoms. When heated to
decomposition it emits acrid smoke and
irritating fumes. | [Hazardous Substances Data]
53-16-7(Hazardous Substances Data) |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Isopropyl acetate-->Acetylacetone-->CHLORIC ACID-->2,4,6-Collidine-->5-ALPHA-ANDROSTANE-->Hydrogen-->Hydrochloric acid-->Palladium-->Bis(4-hydroxyphenyl) Sulfone-->Benzyltrimethylammonium hydroxide-->19-DIOL 3-ACETATE-->PREMARIN-->Estradiol | [Preparation Products]
Oxendolone-->β-Estradiol-->16,17-Epoxy-3,17-dihydroxyestra-1,3,5(10)-triene-3,17-diacetate-->Ethinyl Estradiol-->estra-1,3,5(10),16-tetraene-3,17-diol diacetate-->Estriol-->3-DEOXYESTRONE-->Gestonoronacetat |
| Hazard Information | Back Directory | [Hazard]
A carcinogen (OSHA). | [Potential Exposure]
Synthesized from ergosterol. Used in
combination with progestogen as an oral contraceptive. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit | [Shipping]
UN3249 Medicine, solid, toxic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials. | [Incompatibilities]
May react exothermically with reducing
agents to generate flammable gaseous hydrogen.
Incompatible with oxidizers (chlorates, nitrates, peroxides,
permanganates, perchlorates, chlorine, bromine, fluorine,
etc.); contact may cause fires or explosions. Keep away
from alkaline materials, strong bases, strong acids, oxoacids,
and epoxides. | [Description]
Estrone is one of the three naturally occurring estrogens, the others being estradiol and estriol. Estrone is synthesized from androstenedione by the aromatase enzyme system in the ovaries and placenta, and is also synthesized from estradiol by 17-hydroxy steroid dehydrogenase in the liver.Serum concentrations of estrone in premenopausal women fluctuate according to the menstrual cycle and becomes the most predominant estrogen in postmenopausal women.The binding affinities of estrone to the estrogen receptors α and β are approximately 60% and 37% relative to estradiol. | [Chemical Properties]
Estrone is an odorless white crystalline powder.
Estrone is supplied as a crystalline solid. A stock solution may be made by dissolving the estrone in an organic solvent purged with an inert gas. Estrone is soluble in organic solvents such as DMSO and dimethyl formamide (DMF). The solubility of estrone in these solvents is approximately 20 mg/ml.
Estrone is sparingly soluble in aqueous buffers. For maximum solubility in aqueous buffers, estrone should first be dissolved in DMF and then diluted with the aqueous buffer of choice. Estrone has a solubility of approximately 0.15 mg/ml in a1:5 solution of DMF:PBS (pH 7.2) using this method. We do not recommend storing the aqueous solution for more than one day. | [Originator]
Estrone,Abbott | [Uses]
Estrone is a metabolite of 17β-Estradiol (E888000). During the metabolism, it is in rapid equilibrium with Estriol (E888960) and 17β-Estradiol (E888000) (1). Causes the feminization of male fish at human and animal waste sites (2).��This compound is a contaminant of emerging concern (CECs). Drinking water contaminant candidate list 3 (CCL 3) compound as per United States Environmental Protection Agency (EPA), environmental, and food contaminants. | [Uses]
A metabolite of 17β-Estradiol. | [Uses]
Estrone is a weak form of estrogen and exists as estrone sulphate. Estrone is a luteolytic estrogen produced by the corpus luteum. In the follicle, estrone is synthesized from androstenedione by the action of cytochrome P450 aromatase.
Estrone has been used:
as medium supplement for hormone based degranulation studies of natural killer cells.
as an endocrine disrupting compound for screening bacterial biosensor in toxic water.
as medium component for monitoring fatty acid synthase (FASN) activity in breast adenocarcinoma cell lines. | [Definition]
ChEBI: A 17-oxo steroid that is estra-1,3,5(10)-triene substituted by an hydroxy group at position 3 and an oxo group at position 17. | [Manufacturing Process]
1-Vinyl-1,2,3,4-tetrahydronaphthalene-1,6-diol reacts with 2-
methylcyclopentane-1,3-dione in the presence of Triton B in tert-butanol gives
a good yield of δ1,3,5(10),9(11)-8,14-secoestratetraen-3-ol-14,17-dione, melting
point 124°-126°C (from methanol).
δ1,3,5(10),9(11)-8,14-Secoestratetraen-3-ol-14,17-dione under influence of
hydrochloric acid in tetrahydrofurane cyclises into δ1,3,5(10),8,14-estrapentaen-
3-ol-17-one, melting point 216°-218°C.
δ1,3,5(10),8,14-Estrapentaen-3-ol-17-one is converted to d,l-8-dehydroestrone
by selective hydrogenation with hydrogen, melting point 251°-254°C (from
methanol). Exhaustive hydrogenation of δ1,3,5(10),8,14-estrapentaen-3-ol-17-
one give d,l-8-isoestrone.
d,l-8-Isoestrone in the presence of hydrochloric acid in tetrahydrofurane
isomerizes into d,l-9(11)-dehydroestrone, melting point 262°-265°C (from
alcohol).
Hydrogenation of d,l-9(11)-dehydroestrone in tetrahydrofuran in the presence
of Pd/CaCO3 yields the estrone, melting point 251°-252°C (from acetone). | [Brand name]
Estrogenic Substance (Wyeth); Theelin (Parkdale). | [Therapeutic Function]
Estrogen | [General Description]
Estrone, 3-hydroxyestra-1,3,5(10)-trien-17-one, is less active than estradiol but more active than itsmetabolite, estriol. As the salt of its 3-sulfate ester, estroneis the primary ingredient in conjugated estrogens, USP, andesterified estrogens, USP. Although originally obtainedfrom the urine of pregnant mares (about 10 mg/L), estroneis now prepared synthetically. Estrone itself is not availablein commercial oral formulations, but can be obtained at compounding pharmacies as a topical formulation. Oleoylestrone,the C3 ester of estrone with oleic acid, is in phase IIclinical trials for the treatment of obesity. This acyl estronederivative reduces fat stores by a mechanism not involvingthe ER, although some of the oleoyl-estrone is hydrolyzedto estrone in vivo. | [Biochem/physiol Actions]
Estrone is an agonist for the estrogen receptor. The estradiol to estrone interconversion is favourable in menopause. Oral hormone replacement therapy (HRT) of estradiol-17β increases circulating levels of estrone. | [Synthesis]
Estrone, 3-hydroxyestra-1,3,5(10)-trien-17-one (28.1.9), has been made synthetically
in various ways. According to one of the first and most simple schemes, synthesis
was carried out in the following manner. Condensation of 3-methoxyphenylacetylene
with bicyclohexane-1,5-dione in a Favorskii reaction conditions lead to the corresponding
carbinol (28.1.1). The triple bond was reduced by hydrogen over a palladium catalyst,
forming the tertiary alcohol (28.1.2), which was then dehydrated in acidic conditions to
give the compound (28.1.3). Intramolecular alkylation of this compound in the presence of
anhydrous aluminum chloride formed a tetracyclic ketone (28.1.4), which during condensation
with benzaldehyde was transformed into an eneone (28.1.5). This was methylated
at the |?-position relative to the keto-group by methyl iodide in the presence of potassium
tert-butylate, and the resulting compound (28.1.6) underwent ozonolysis, forming the
dicarboxylic acid (28.1.7). Cyclization of this compound to a cyclopentanone derivative
lead to the formation of methyl ester of the desired estrone (28.1.8), and demethylation of the phenolic hydroxyl group by hydrobromic acid formed the desired estrone (28.1.9).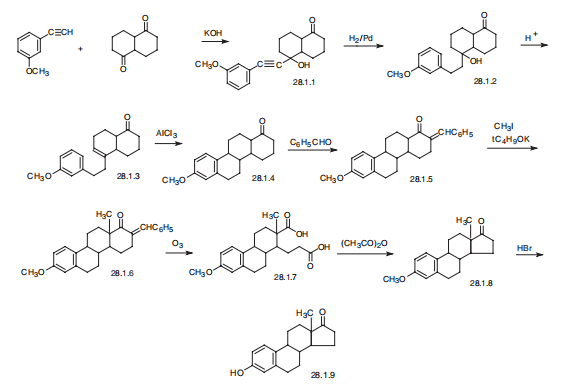
| [storage]
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, well-ventilated area. A regulated, marked area should be established where this chemical is handled, used, or stored incompliance with OSHA Standard 1910.1045. | [Purification Methods]
Purify estrone by chromatography on silica gel, eluting with 2:1 hexane/EtOAc and recrystallising from EtOH or Et2O/EtOH. [Danishefsky & Cain J Am Chem Soc 98 4975 1976.] The acetate [901-93-9] crystallises from EtOH with m 125-127o. [Beilstein 8 III 1171.] |
|
|