| Identification | More | [Name]
Adenosine | [CAS]
58-61-7 | [Synonyms]
(2R,3R,4S,5R)-2-(6-AMINO-PURIN-9-YL)-5-HYDROXYMETHYL-TETRAHYDRO-FURAN-3,4-DIOL
9-BETA-D-RIBOFURANOSYLADENINE
ADENINE-9-BETA-D-RIBOFURANOSIDE
ADENINE-9-D-RIBOFURANOSIDE
ADENINENUCLEOSIDE
ADENINE RIBOSIDE
ADENOSINE
AR
D-ADENOSINE
6-amino-9-beta-d-ribofuranosyl-9h-purine
6-Amino-9beta-D-ribofuranosyl-9H-purine
9-beta-D-Ribofuranosidoadenine
9-beta-D-Ribofuranosyl-9H-purin-6-amine
9H-Purin-6-amine, 9-beta-d-ribofuranosyl-
Adenocard
Adenocor
Adenoscan
Adenosin
beta-Adenosine
beta-d-Adenosine | [EINECS(EC#)]
200-389-9 | [Molecular Formula]
C10H13N5O4 | [MDL Number]
MFCD00005752 | [Molecular Weight]
267.24 | [MOL File]
58-61-7.mol |
| Chemical Properties | Back Directory | [Appearance]
White crystalline powder | [Melting point ]
234-236 °C (lit.) | [alpha ]
D11 -61.7° (c = 0.706 in water); 9D -58.2° (c = 0.658 in water) | [Boiling point ]
410.43°C (rough estimate) | [density ]
1.3382 (rough estimate) | [refractive index ]
1.7610 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
Slightly soluble in water, soluble in hot water, practically insoluble in ethanol (96 per cent) and in methylene chloride. It dissolves in dilute mineral acids. | [form ]
Crystalline Powder | [pka]
3.6, 12.4(at 25℃) | [color ]
White | [PH]
3.59;12.5 | [Stability:]
Stable. Incompatible with strong oxidizing agents. | [biological source]
synthetic (organic) | [Optical Rotation]
[α]20/D 70±3°, c = 2% in 5% NaOH | [Water Solubility ]
Soluble in water, ammonium hydroxide and dimethyl sulfoxide. Insoluble in ethanol. | [λmax]
257 (pH 1);260 (pH 6) | [Merck ]
14,153 | [BRN ]
93029 | [Cosmetics Ingredients Functions]
SKIN CONDITIONING | [InChI]
1S/C10H13N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h2-4,6-7,10,16-18H,1H2,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | [InChIKey]
OIRDTQYFTABQOQ-KQYNXXCUSA-N | [SMILES]
Nc1ncnc2n(cnc12)[C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O | [LogP]
-0.755 (est) | [Uses]
adenosine is an amino acid. Studies indicate anti-wrinkle and skinsmoothing capacities. Although little is written about its direct skin benefit, adenosine plays an important role in biochemical processes. As adenosine triphosphate (ATP) and adenosine diphosphate (ADP), it is involved in energy transfer, and as cyclic adenosine monophosphate (cAMP) in signal transduction. | [CAS DataBase Reference]
58-61-7(CAS DataBase Reference) | [NIST Chemistry Reference]
adenosine(58-61-7) | [EPA Substance Registry System]
Adenosine (58-61-7) |
| Hazard Information | Back Directory | [Description]
Adenosine(58-61-7) is a nucleoside composed of a molecule of adenine attached to a ribose sugar molecule (ribofuranose) moiety via a β-N9-glycosidic bond. Adenosine works in the energy transfer-as adenosine triphosphate (ATP) and adenosine diphosphate (ADP)-as well as in signal transduction as cyclic adenosine monophosphate, cAMP. It shows neuromodulatory, cytoprotective, anti-inflammatory and cardioprotective actions.
| [Chemical Properties]
Adenosine is an important nucleoside composed of adenine and ribose. White, crystalline, odorless powder, mild, saline, or bitter taste, quite soluble in hot water, practically insoluble in alcohol. Formed by isolation following hydrolysis of yeast nucleic acid. | [Application]
Adenosine can serve as a diagnostic or therapeutic agent.
Diagnostically, adenosine is one pharmaceutical agent used in a
myocardial perfusion stress imaging study for its vasodilatory effects.
Therapeutically, adenosine is used for its antiarrhythmic properties in
supraventricular tachycardia (SVT) and can function as a diagnostic
tool, depending on the type of SVT. | [Definition]
ChEBI: Adenosine is a ribonucleoside composed of a molecule of adenine attached to a ribofuranose moiety via a beta-N(9)-glycosidic bond. It has a role as an anti-arrhythmia drug, a vasodilator agent, an analgesic, a human metabolite and a fundamental metabolite. It is a purines D-ribonucleoside and a member of adenosines. It is functionally related to an adenine. | [Brand name]
Adenocard (Astellas); Adenoscan (Astellas). | [Origin]
Adenosine may be generated intracellularly in the central nervous system from degradation of AMP or from the hydrolysis of S-adenosyl homocysteine, and then exit via bi-directional nucleoside transporters, or extracellularly by the metabolism of released nucleotides. Inactivation of extracellular adenosine occurs by transport into neurons or neighboring cells, followed by either phosphorylation to AMP by adenosine kinase or deamination to inosine by adenosine deaminase[1]. | [Biological Functions]
Adenosine regulates multiple physiological and pathophysiological processes, by acting both through G-protein coupled adenosine receptors and intracellularly. It modulates neuronal plasticity, astrocytic activity , learning and memory, food intake, motor function, sleep/wake cycle, pain, immunosupression, proliferation, and aging. Adenosine is involved in ischemia and stroke, epilepsy, and neurodegenerative pathologies such as Parkinson's disease (PD), Alzheimer's disease (AD), amyotrophic lateral sclerosis (ALS), and Huntington's disease (HD). Extracellular adenosine, interacting with P1 receptors (A1R, A2AR, A2BR, and A3R) regulates metabolism through different signaling pathways[1].
| [Biological Functions]
[1] Mercedes Garcia-Gil. “Metabolic Aspects of Adenosine Functions in the Brain.” Frontiers in Pharmacology (2021): 672182. | [General Description]
Adenosine is a purine nucleoside and a building block of RNA and many other biomolecules such as adenosine triphosphate and nicotinamide adenine dinucleotide. In the extracellular space, ecto-5′-nucleotidase (CD73) dephosphorylates adenosine triphosphate (ATP) to produce adenosine. Adenosine has four receptors namely A1R, A2AR A2BR and A3R. Adenosine plays a key role in the osteogenic differentiation. A1R induces osteoclast differentiation and A2AR induces osteoblast differentiation. | [Biological Activity]
Neurotransmitter that acts as the preferred endogenous agonist at all adenosine receptor subtypes. | [Biochem/physiol Actions]
Endogenous neurotransmitter at adenosine receptors. Cardioprotective effects may relate to activation of A1 adenosine receptors. The antiplatelet and anti?inflammatory actions of adenosine appear to be mediated via the A2 adenosine receptor. In contrast, adenosine appears to be a pro-inflammatory mediator in asthma and chronic obstructive pulmonary disease (COPD). | [Clinical Use]
Adenosine (Adenocard) is an endogenous nucleoside
that is a product of the metabolism of adenosine triphosphate.
It is used for the rapid termination of supraventricular
arrhythmias following rapid bolus dosing.
Adenosine is approved for the acute management and
termination of supraventricular tachyarrhythmias, including A-V nodal reentrant tachycardia and A-V reciprocating
tachycardia. Adenosine may be helpful in the
diagnosis of atrial flutter. | [Side effects]
Adverse reactions to the administration of adenosine are
fairly common; however, the short half-life of the drug
limits the duration of such events.The most common adverse
effects are flushing, chest pain, and dyspnea.
Adenosine may induce profound bronchospasm in patients
with known reactive airway disease. The mechanism
for bronchospasm is unclear, and the effect may last
for up to 30 minutes despite the short half-life of the drug. | [Drug interactions]
Metabolism of adenosine is slowed by dipyridamole, indicating
that in patients stabilized on dipyridamole the
therapeutically effective dose of adenosine may have to
be increased. Methylxanthines antagonize the effects of
adenosine via blockade of the adenosine receptors. | [Metabolism]
It is impossible to study adenosine in classical
pharmacokinetic studies, since it is present in various
forms in all the cells of the body. An efficient salvage
and recycling system exists in the body, primarily in
erythrocytes and blood vessel endothelial cells. The half�life in vitro is estimated to be less than 10 seconds, and
may be even shorter in vivo. | [storage]
-20°C | [Purification Methods]
Crystallise adenosine from distilled water and dry it at 110o. It has been purified via the picrate, where ethanolic picric acid is added to adenosine and the picrate is filtered off and recrystallised from EtOH. It has m 180-185o(dec). Adenosine is recovered by dissolving 0.4g of the picrate in 80mL of hot H2O, treated with a small quantity of Dowex 1 anion exchange resin in the chloride form, and the resin is filtered off. The filtrate is treated with more resin and filtered again. One equivalent of aqueous NaOH is added to the colourless filtrate which is evaporated to 4mL and cooled to give 0.176g of adenosine m 236o. [Davoll et al. J Chem Soc 967 1948, Davoll & Lowy J Am Chem Soc 73 1650 1951, Beilstein 26 III/IV 3598.] | [Precautions]
Patients with second- or third-degree A-V block should
not receive adenosine. As indicated previously, the use
of adenosine in asthmatic patients may exacerbate the
asthmatic symptoms. |
| Questions And Answer | Back Directory | [Defination]
Adenosine is a natural nucleotide, which is the intermediate product of metabolism, chemically 6-amino-9-beta-D-ribofuranosyl-9-H-purine. Adenosine is one of the important active components in the body, helps in cellular energy transfer by forming molecules like adenosine triphosphate (ATP) and adenosine diphosphate (ADP). It also plays a role in signaling various pathways and functions in the body by forming signally molecules like cyclic adenosine monophosphate (cAMP).
| [In the body]
Adenosine in the body
Function
Brain
Promoting sleep and suppresses arousal acting as a central nervous system depressant.
Heart
Causing dilation of the coronary blood vessels that Improving blood circulation to the heart; Increasing the diameter of blood vessels in the peripheral organs; Decreasing heart rate
Blood
Broken down by adenosine deaminase. By taking medicine like Dipyridamole(inhibitor of adenosine deaminase), it can improve blood flow through the coronary blood vessels that supply the heart muscles.
Kidneys, lungs and liver
In the kidneys adenosine decreases renal blood flow and decrease the production of rennin from the kidneys. In the lungs it causes constriction of airways and in the liver it leads to constriction of blood vessels and increases breakdown of glycogen to form glucose.
| [Medical uses]
Adenosine has a role in the expansion of coronary artery and myocardial contractility, is clinically applied in the treatment of angina, hypertension, cerebrovascular disorders, stroke sequelae, muscular atrophy, etc. It is also given intravenously (by IV) for treating supraventricular tachycardia and Tl myocardial imaging. It is also used for cardiac stress tests.
Side effects:
Since the half-life of this compound is less than 10 seconds, its side effects are usually transient. However, side effects are common, and include flushing, headache, chest discomfort, bronchoconstriction, and occasionally hypotension. Hepatic and renal failure and other drugs except dipyridamole seem to have little effect on the action of adenosine.
Adenosine dose
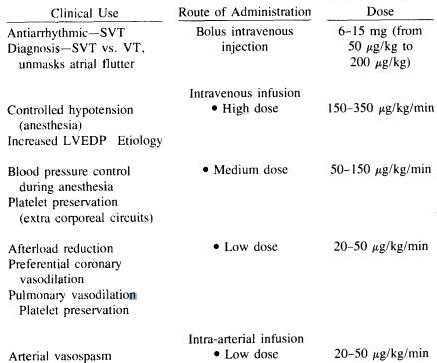
| [Mechanism of action]
Its function is realized through the activation of the adenosine receptor (A receptor). Adenosine activates G protein coupled potassium channels by binding to the A receptor which makes increasing the outflow of K+ and cell membrane hyperpolarization so as to decrease the automaticity in the atrium, sinoatrial node and atrioventricular node. It can also significantly increase the level of cGMP , prolong ERP of the atrioventricular node and slowing of atrioventricular, depress sympathetic nervous or early and delayed after depolarization induced by isoproterenol and then plays an effective role in arrhythmia. This product has not been classified in I~IV anti arrhythmia medicine.
Adenosine receptor
- A1 receptors, which are found in cardiomyocytes and which are responsible for the inhibition of adenylyl cylase activity which lowers cyclic adenosine monophosphate (AMP) results in sinus slowing, increase in AV node conduction delay, and antagonism of the effects of catecholamines;
- A2 receptors, which are found in endothelial cells and vascular smooth muscle and are responsible for the enhancement of adenylyl cylase activity and increased cyclic AMP which relaxes smooth muscle. Both negative chronotropic and dromotropic effects of adenosine are cyclic AMP independent (direct action) as well cyclic AMP dependent (indirect action).
|
|
|