| Identification | More | [Name]
tert-Butylchlorodiphenylsilane | [CAS]
58479-61-1 | [Synonyms]
T-BUTYLCHLORODIPHENYLSILANE
T-BUTYL DIPHENYLCHLOROSILANE
T-BUTYLDIPHENYLSILYL CHLORIDE
TERT-BUTYLCHLORODIPHENYLSILANE
TERT-BUTYLDIPHENYLCHLOROSILANE
TERT-BUTYLDIPHENYLSILYL CHLORIDE
TIMTEC-BB SBB009012
butylchlorodiphenylsilane
CB2805
chloro(1,1-dimethylethyl)diphenyl-silan
Tert-Butylchloridediphenylsilane
tert-Butylsiphenylchlorosilane
tert-Butyl Diphenylchlorosilane (TBDPSCl)
TERT-BUTYLCHLORODIPHENYLSILANE 98%
TERT-BUTYLDIPHENYL CHLOROSILANE, TBDPSCL, REDISTILLED
TBDPSCl
tert-Butyldiphenylchlorosilane,97%
tert-Butyldiphenylchlorosilane(tert-Butylchlorodiphenylsilane)
tert-BUTYLDIPHENYLCHLOROSILANE 97%
Silane, chloro(1,1-dimethylethyl)diphenyl- | [EINECS(EC#)]
261-282-0 | [Molecular Formula]
C16H19ClSi | [MDL Number]
MFCD00000497 | [Molecular Weight]
274.86 | [MOL File]
58479-61-1.mol |
| Chemical Properties | Back Directory | [Appearance]
Colorless-light yellow to brown liquid | [Boiling point ]
90 °C0.01 mm Hg(lit.)
| [density ]
1.057 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.568(lit.)
| [Fp ]
>230 °F
| [storage temp. ]
Store at 5°C | [solubility ]
miscible in most organic solvents. | [form ]
Liquid | [color ]
Clear colorless to yellow or slightly brown | [Specific Gravity]
1.074 | [Water Solubility ]
reacts | [Hydrolytic Sensitivity]
7: reacts slowly with moisture/water | [Sensitive ]
Moisture Sensitive | [BRN ]
644023 | [InChI]
1S/C16H19ClSi/c1-16(2,3)18(17,14-10-6-4-7-11-14)15-12-8-5-9-13-15/h4-13H,1-3H3 | [InChIKey]
MHYGQXWCZAYSLJ-UHFFFAOYSA-N | [SMILES]
CC(C)(C)[Si](Cl)(c1ccccc1)c2ccccc2 | [CAS DataBase Reference]
58479-61-1(CAS DataBase Reference) | [NIST Chemistry Reference]
Silane, chloro(1,1-dimethylethyl)diphenyl-(58479-61-1) | [EPA Substance Registry System]
58479-61-1(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
C,Xi | [Risk Statements ]
R14:Reacts violently with water.
R34:Causes burns.
R37:Irritating to the respiratory system.
R29:Contact with water liberates toxic gas.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S8:Keep container dry . | [RIDADR ]
UN 2987 8/PG 2
| [WGK Germany ]
3
| [F ]
10 | [Hazard Note ]
Irritant/Corrosive | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
II | [HS Code ]
29310095 | [Storage Class]
8A - Combustible corrosive hazardous materials | [Hazard Classifications]
Eye Dam. 1
Skin Corr. 1B
STOT SE 3 |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
2-Chloro-2-methylpropane-->Dichlorodiphenylsilane-->Hexane-->Tetrahydrofuran-->Magnesium | [Preparation Products]
3-AMINO-2,2-DIMETHYL-4-OXO-AZETIDINE-1-SULFONIC ACID-->2-(t-Butyldiphenylsilanyloxy)Ethanol-->(S)-4-BOC-MORPHOLINE-3-CARBOXYLIC ACID-->Fluvastatin sodium salt-->4-Pentylcyclohexanone-->Benzene, 1,1'-[(2-bromoethoxy)(1,1-dimethylethyl)silylene]bis--->(S)-2-(TERT-BUTOXYCARBONYLAMINO)-3-(TERT-BUTYLDIPHENYLSILYLOXY)PROPANOIC ACID-->(But-3-en-1-yloxy)(tert-butyl)diphenylsilane-->Benzene, 1,1'-[(1,1-dimethylethyl)(2-propen-1-yloxy)silylene]bis--->2-(2-((tert-butyldiphenylsilyl)oxy)ethoxy)ethanol(WXPC0004)-->6-O-(TERT-BUTYLDIPHENYLSILYL)-D-GLUCAL-->METHYL-(6R)-((T-BUTYL) DIPHENYLSILYLOXY)-(2E,4E,8Z)-TETRADECATRIENOATE-->2,6-DI([1-(TERT-BUTYL)-1,1-DIPHENYLSILYL]OXY)-9,10-DIHYDROANTHRACENE-9,10-DIONE-->tert-Butyl(3-butynyloxy)diphenylsilane |
| Hazard Information | Back Directory | [Chemical Properties]
Tert-Butylchlorodiphenylsilane is a colorless to pale brown oily liquid with pungent odor, may be used as silylating agent for derivatization of alcohols, ketones, carboxylic acids, amines, amides and mercaptanes selectively into functional groups in different sterical environments.
| [Physical properties]
colorless liquid, bp 93–95°C/0.015 mmHg;
n20
D 1.5680; d 1.057 g cm?3. | [Uses]
Several newmethods have been developed
for using the reagent to protect primary and secondary alcohols
as their TBDPS ethers. In the presence of ammonium nitrate or
ammonium perchlorate, reaction between TBDPS-Cl and a primary
alcohol, such as benzyl alcohol, in DMF provided excellent
yields of the corresponding silyl ethers in just 15 min (eq 1).19
When silver nitrate was used as promoter, the reactions gave
inferior yields under otherwise identical conditions.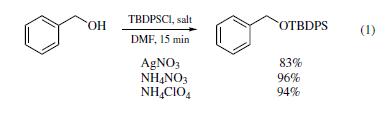
When TBDPS-Cl is used to react with hemiacetals, it converts
hemiacetals into ring-opened silyl ether carbonyl compounds, instead
of mixed acetals.Presumably, the sizable TBDPS group
presents too much steric hindrance for the formation of the corresponding
mixed silyl acetals. | [Application]
tert-Butylchlorodiphenylsilane (TBDPSCl) is a good reagent for chemical reaction, widely used in the fields of organic synthesis, thin film materials and medicine. The main applications are as follows:
(1) It can be used for the preparation of T - SSBR (SSBR end - capped by tert - Butylchlorodiphenylsilane). Since TBCSi end sealing can fix the free chain ends of T-SSBR (that is, reduce the frictional loss of molecular chains and generate a greater orientation degree in the force field), and adsorb carbon black, the performance of T-SSBR composites is superior to that of SSBR. Moreover, the performance of styrene-TBCSi terminated T-SSBR (TS-SSBR) is much better than that of butadiene-TBCSi terminated T-SSBR (TB-SSBR). Therefore, the former is applicable to the tread of green tires.[1]
(2) TBDPSCl can be used to prepare fluorene-based polyimides (Si-PIs) containing silyl ether groups by silylation with hydroxyl-containing polyimides (OH-PIs). The optical, dielectric and solubility properties of the modified Si-PI films are simultaneously improved compared to the precursor OH-PI films. The modified Si–PI films demonstrate a meaningful enhancement in the transmittances at a wavelength of 400 nm (T400) to 74–81% from 42 to 55% of OH–PI films and the regeneration of fluorescence characteristics. The dielectric constant and loss of Si–PI films are also obviously reduced to 2.63–2.75 and 0.0024–0.0091 at 1 kHz from 4.19 to 4.78 and 0.0173–0.0295 of OH–PI films, respectively, due to substituted with the bulky nonpolar TBDPS groups to increase the free volume and hydrophobicity of Si–PI films. The solubility of Si–PIs in low- or nonpolar solvents (such as CHCl3, CH2Cl2, acetone, and toluene) is significantly improved. Furthermore, Si–PI films still maintain relatively good thermal properties with the 5% weight loss temperature (T5%) in the range 470–491 °C under a nitrogen atmosphere and the glass transition temperature (Tg) in the range 245–308 °C.[2] | [Preparation]
a dry 1 L, three-necked round bottomed
flask is equipped with a magnetic stirring bar, a 500mL equalizing
dropping funnel fitted with a rubber septum, a reflux condenser,
and nitrogen inlet tube. The flask is flushed with nitrogen,
then charged with 127 g (0.5 mol) of diphenyldichlorosilane
in 300mL of redistilled pentane. A solution of tbutyllithium
in pentane (500 mL, 0.55 mol), is transferred under
nitrogen pressure to the dropping funnel using a stainless steel,
double-tip transfer needle. This solution is slowly added to the
contents of the flask and when the addition is complete, the
mixture is refluxed 30 h under nitrogen with stirring. The suspension
is allowed to cool to rt, the precipitated lithium chloride
is rapidly filtered through a pad of Celite, and the latter
is washed with 200mL of pentane. The solvent is removed by
evaporation, and the colorless residue is distilled through a short
(10 cm), Vigreux column, to give 125–132 g of the colorless title
compound. | [reaction suitability]
reagent type: derivatization reagent
reaction type: Silylations | [Synthesis]
Example 2: To a 500 mL four-necked flask equipped with a reflux condenser, a charging funnel, a thermometer, and a stir bar was added 12.2 g (0.5 mol) of magnesium under nitrogen protection. After drying, tert-butyl Grignard reagent was prepared by adding 250 mL of tetrahydrofuran and 46.3 g (0.5 mol) of chlorinated tert-butane by stirring. At room temperature, 0.45 g (0.005 mol) of copper cyanide was added to the reaction system, followed by the slow dropwise addition of 126.6 g (0.5 mol) of diphenyl dichlorosilane under stirring. During the reaction, the system temperature was raised to 50°C. After reflux heating and stirring for 5 h, the reaction mixture was diluted by adding 100 mL of hexane and the insoluble material was removed by filtration. After distillation to remove the solvent, it was purified by vacuum distillation to give 103 g (75% yield) of tert-butyl diphenylchlorosilane. | [Purification Methods]
Purify it by repeated fractional distillaton. It is soluble in DMF and pentane [Hanessian & Lavalee Can J Chem 53 2975 1975, Robl et al. J Med Chem 34 2804 1991]. [Beilstein 4 IV 4076 for tert-butylchlorodimethylsilane.] | [References]
[1] LEI WANG . Study on the structure and properties of SSBR with large-volume functional groups at the end of chains[J]. Polymer, 2010, 51 9: Pages 2084-2090. DOI:10.1016/j.polymer.2010.03.006.
[2] YANCHENG WU*; Shumei L, JIANQING ZHAO; Simultaneously Improving the Optical, Dielectric, and Solubility Properties of Fluorene-Based Polyimide with Silyl Ether Side Groups[J]. ACS Omega, 2022, 7 14: 11939-11945. DOI:10.1021/acsomega.2c00069. |
|
|