| Identification | More | [Name]
Uracil | [CAS]
66-22-8 | [Synonyms]
2,4(1H,3H)-PYRIMIDINEDIONE
2,4-DIHYDROXYPYRIMIDINE
2,4-DIOXOPYRIMIDINE
2,4-PYRIMIDINEDIOL
2-HYDROXY-4(1H)-PYRIMIDINONE
CCTGCCCTGUGCAGCTGTGGG
Cellocidin
Cellomate
PYRIMIDINE-2,4(1H,3H)-DIONE
PYRIMIDINE-2,4-DIOL
1H-Pyrimidine-2,4-dione
2,(1H,3H)-Pyriminedione
2,4-Dioxypyrimidine
2,4-Pyrimidinedione
2,6-Dihydroxypyrimidine
2-Hydroxy-4(3H)-pyrimidinone
4-Hydroxy-2(1H)-pyrimidinone
Hybar X
hybarx
Pirod | [EINECS(EC#)]
200-621-9 | [Molecular Formula]
C4H4N2O2 | [MDL Number]
MFCD00006016 | [Molecular Weight]
112.09 | [MOL File]
66-22-8.mol |
| Chemical Properties | Back Directory | [Appearance]
white powder | [Melting point ]
>300 °C (lit.) | [Boiling point ]
209.98°C (rough estimate) | [density ]
1.4421 (rough estimate) | [refractive index ]
1.4610 (estimate) | [storage temp. ]
2-8°C | [solubility ]
Aqueous Acid (Slightly), DMSO (Slightly, Heated, Sonicated), Methanol (Slightly) | [form ]
Crystalline Powder | [pka]
9.45(at 25℃) | [color ]
White to slightly yellow | [Stability:]
Stable. Incompatible with strong oxidizing agents. | [biological source]
synthetic (organic) | [Water Solubility ]
SOLUBLE IN HOT WATER | [Detection Methods]
HPLC,NMR | [Merck ]
14,9850 | [BRN ]
606623 | [Cosmetics Ingredients Functions]
SKIN CONDITIONING | [InChI]
1S/C4H4N2O2/c7-3-1-2-5-4(8)6-3/h1-2H,(H2,5,6,7,8) | [InChIKey]
ISAKRJDGNUQOIC-UHFFFAOYSA-N | [SMILES]
O=C1NC=CC(=O)N1 | [LogP]
-1.037 (est) | [CAS DataBase Reference]
66-22-8(CAS DataBase Reference) | [NIST Chemistry Reference]
Uracil(66-22-8) | [EPA Substance Registry System]
66-22-8(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
2
| [RTECS ]
YQ8650000
| [TSCA ]
Yes | [HS Code ]
29335900 | [Storage Class]
11 - Combustible Solids | [Safety Profile]
Moderately toxic by
intraperitoneal route. An experimental
teratogen. Experimental reproductive
effects. Questionable carcinogen with
experimental tumorigenic data. Mutation
data reported. When heated to
decomposition it emits toxic fumes of NOx. |
| Hazard Information | Back Directory | [Description]
Uracil is a pyrimidine base and a fundamental component of RNA where it binds to adenine via hydrogen bonds.1 It is converted into the nucleoside uridine through the addition of a ribose moiety, then to the nucleotide uridine monophosphate by the addition of a phosphate group. | [Chemical Properties]
Crystalline needles. Soluble in hot water, ammonium hydroxide, and other alkalies; insoluble in alcohol and ether.
| [Definition]
A nitrogenous base that
is found in RNA, replacing the thymine of
DNA. It has a pyrimidine ring structure. | [Definition]
ChEBI: A common and naturally occurring pyrimidine nucleobase in which the pyrimidine ring is substituted with two oxo groups at positions 2 and 4. Found in RNA, it base pairs with adenine and replaces thymine during DNA transcription. | [Definition]
uracil: A pyrimidine derivative andone of the major component bases ofnucleotides and the nucleic acidRNA. | [General Description]
Certified pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. Uracil is one of the nucleotide bases of RNA. It is the precursor of DNA′s thymine. It acts like a carrier of genetic data and is hooked up with a ribose and three phosphates to form a ribonucleoside triphosphate once a human body produces nucleotides. | [Purification Methods]
Uracil crystallises from water (m 339-341o) and m 338o after sublimation in high vacuum. Its solubility in H2O at 20o is 1g/300mL. [Beilstein 24 H 312, 24 I 312, 24 II 169, 24 III/IV 1193.] |
| Questions And Answer | Back Directory | [Organic alkali]
Uracil is an organic alkali, and is one of the four major bases in RNA. It is a major component of the pyrimidine composition in ribonucleic acid (RNA) as well as in various kinds of uridines. It can connect with ribose to generate UMP whose triphosphate compound being UTP. UTP is the precursor form of uracil in RNA biosynthesis. UTP also acts as a coenzyme to be involved in the biosynthesis of certain sugars. Uracil can block the degradation effect of tegafur, and thus increasing the concentration of fluorouracil which enhance the anti-cancer effects. Fluorouracil has similar clinical indications as uracil. It is mainly used for treating digestive cancer, breast cancer and thyroid cancer. Combination with mitomycin has a good efficacy on treating advanced gastric cancer. Laboratory synthesizes uracil through the cyclization reaction between ethyl malonyl and urea for pharmaceutical and biochemical research.
Uracil has tautomerism effect:
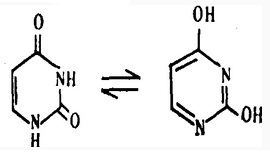
Keto (2,4-2 CPCC) enol (2,4-2-hydroxy pyrimidine) in mainly exist in the form of ketone inside biological cells.
Nature uracil is presented mainly in marine organisms, particulate matter and sea lysate. It is treated as life indicator in the field of organic geochemistry.
Pyrimidine refers to the hexaheterocyclic compound with two nitrogen atoms in 1,3-position of the benzene ring, and it, together with pyridazine and pyrazine, are isomers of each other. Pyrimidine has a unique UV spectrum due to the presence of conjugated double bonds in its structure. Pyrimidine has a lower basicity and a weaker lectrophilic substitution reaction than pyridine. But it is more prone to have nucleophilic substitution. Derivatives of pyrimidine are widely distributed in nature, including vitamin B1, uracil, thymine, and cytosine which all containing a pyrimidine structure.
| [Fluorouracil]
Fluorouracil, briefly referred as FU, is currently one of the most commonly used anti-cancer drug. It is white crystals with pKa = 8.1, m.p.282~283 °C. It is slightly soluble in water (12mg/ml at 25 °C) and ethanol, but insoluble in chloroform and ether. It is easily soluble in diluted acid and alkali. It is hydrolyzed in the presence of strong base but is stable in normal saline. Due to the introduction of a strong electrically fluorine atoms, the acidity of Fu is 30 times higher than its parent, uracil. The injection of Fu usually is an aqueous solution with pH 9.0 adjusted by sodium hydroxide. It is sensitive to light and easy to crystallize when stored at low temperatures or prolonged room temperature.
According to the stronger ability of tumor tissue of rats in utilizing pyrimidine than normal tissue n, in 1957, Duschinsky and Heidelbergere designed and replace the 5-hydrogen in uracil to fluorine with similar size and generated Fu, as an anti-metabolite of uracil to achieve selective anticancer effects. FU has inhibitory effects on many kinds of animal transplanted tumors such as mouse leukemia L1210, L615, and adenocarcinoma 755. Tumor cells has no cross-resistance to it and other commonly used anti-cancer drugs such as cytarabine, methotrexate, mercaptopurine, cyclophosphamide, and carmustine.
FU is converted into 5-fluoro-deoxy-uridine monophosphate (FDUMP) and 5-fluorouracil nucleoside triphosphate (FUTP) in tissues. FDUMP inhibits the thymidylate synthase (TS) via forming compound with TS and 5,10-methenyltetrahydrofolate, thus resulting in a lack of intracellular thymine nucleotide and further inhibition of DNA synthesis, finally leading to cell death. On the other hand, FUTP is incorporated into RNA as the substrate of RNA polymerase substrate and affect the normal synthesis and function of RNA. In tissue culture, supplement of thymidine (TdR) can reverse the FU cytotoxicity, so that it has been realized for many years that the impact on DNA is the primary growth-inhibitory mechanism of FU. However, it was found that TdR didn’t completely reverse the cytotoxicity of FU, and the combination of FU and TdR significantly improved the FU’s incorporation into RNA and its anti-cancer effect. After culturing together of L1210 leukemia cells with 6-H3-5FU for 22 hours, it was found the existence of 80 fmol of FDUMP-TS-5,10CH2-H4 folic acid complexes in 106 cell while 400 fmol of FU bound to RNA. This emphasizes the importance of FU’s incorporation into RNA FU for its anti-tumor effect. FU is a cell cycle-specific drug which playing the significant role at S-phase.
Reference: China Medical Encyclopedia Editing Committee; editor: Liang Huang; Chinese Medical Encyclopedia.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
| [Chemical property]
White or light yellow crystalline needle. Melting point 338 °C; easily soluble in water, soluble in diluted ammonia, slightly soluble in cool water, insoluble in alcohol and ether.
| [Uses]
For biochemical research, drugs synthesis; being used as pharmaceutical intermediates, also used in organic synthesis
| [Production methods]
It is produced through the reaction of malate, sulfuric acid and urea.
|
|
|