| Identification | More | [Name]
5-Fluorouracil | [CAS]
51-21-8 | [Synonyms]
1-FLUORO-1H-PYRIMIDINE-2,4-DIONE
2,4-DIHYDROXY-5-FLUOROPYRIMIDINE
5-FLUORO-1H-PYRIMIDINE-2,4-DIONE
5-FLUORO-2,4(1H,3H)-PYRIMIDINEDIONE
5-FLUORO-2,4-DIHYDROXYPYRIMIDINE
5-fluoro-2,4-pyrimidinedione
5-FLUOROPYRIMIDINE-2,4(1H,3H)-DIONE
5-Fluoropyrimidine-2,4-dione
5-FLUOROURACIL
5-FU
FLUOROURACIL
FU
[180]-5-Fluorouracil
2,4(1H,3H)-Pyrimidinedione, 5-fluoro-
2,4-dioxo-5-fluoropyrimidine
3h)-pyrimidinedione,5-fluoro-4(1h
5-faracil
5-Flouracyl
5-fluor-2,4(1h,3h)-pyrimidindion
5-Fluor-2,4-dihydroxypyrimidin | [EINECS(EC#)]
200-085-6 | [Molecular Formula]
C4H3FN2O2 | [MDL Number]
MFCD00006018 | [Molecular Weight]
130.08 | [MOL File]
51-21-8.mol |
| Chemical Properties | Back Directory | [Appearance]
Fluorouracil is a white crystalline solid.
Practically odorless. | [Melting point ]
282-286 °C (dec.) (lit.) | [Boiling point ]
190-200°C/0.1mmHg | [density ]
1.4593 (estimate) | [storage temp. ]
Store at 0-5 | [solubility ]
H2O: 10 mg/mL, clear
| [form ]
powder
| [pka]
pKa 8.0±0.1 (H2O) (Uncertain);3.0±0.1(H2O) (Uncertain) | [color ]
white
| [PH]
4.3-5.3 (10g/l, H2O, 20℃) | [Stability:]
Stable. Light sensitive. Combustible. Incompatible with strong oxidizing agents, strong bases. | [Water Solubility ]
12.2 g/L 20 ºC | [Sensitive ]
Air Sensitive | [Usage]
A potent antineoplastic agent in clinical use. Also an inhibitor of DNA synthesis | [Detection Methods]
HPLC,NMR | [Merck ]
14,4181 | [BRN ]
127172 | [Major Application]
diagnostic assay manufacturing
hematology
histology | [InChI]
1S/C4H3FN2O2/c5-2-1-6-4(9)7-3(2)8/h1H,(H2,6,7,8,9) | [InChIKey]
GHASVSINZRGABV-UHFFFAOYSA-N | [SMILES]
FC1=CNC(=O)NC1=O | [CAS DataBase Reference]
51-21-8(CAS DataBase Reference) | [IARC]
3 (Vol. 26, Sup 7) 1987 | [NIST Chemistry Reference]
2,4-Pyrimidinedione, 5-fluoro-(51-21-8) | [EPA Substance Registry System]
51-21-8(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,T,C,Xi | [Risk Statements ]
R22:Harmful if swallowed.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S36:Wear suitable protective clothing .
S36/37:Wear suitable protective clothing and gloves .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S22:Do not breathe dust . | [RIDADR ]
UN 2811 6.1/PG 3
| [WGK Germany ]
3
| [RTECS ]
YR0350000
| [F ]
10-23 | [Hazard Note ]
Irritant/Highly Toxic | [TSCA ]
T | [HazardClass ]
6.1 | [PackingGroup ]
III | [HS Code ]
29335995 | [Storage Class]
6.1C - Combustible acute toxic Cat.3
toxic compounds or compounds which causing chronic effects | [Hazard Classifications]
Acute Tox. 3 Oral
Carc. 2 | [Safety Profile]
Poison by ingestion,
intraperitoneal, subcutaneous, and
intravenous routes. Moderately toxic by
parented and rectal routes. Experimental
teratogenic and reproductive effects. Human
systemic effects: EKG changes, bone
marrow changes, cardiac, pulmonary, and
gastrointestinal effects. Human mutation
data reported. A human skin irritant.
Questionable carcinogen. When heated to decomposition it emits very toxic fumes of
Fand NOx. | [Hazardous Substances Data]
51-21-8(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: 230 mg/kg |
| Hazard Information | Back Directory | [General Description]
White to nearly white crystalline powder; practically odorless. Used as an anti neoplastic drug, chemosterilant for insects. | [Reactivity Profile]
FLUOROURACIL(51-21-8) may be sensitive to prolonged exposure to light. Solutions discolor on storage. This chemical can react with oxidizing agents and strong bases. Incompatible with methotrexate sodium. | [Air & Water Reactions]
Insoluble in water. | [Hazard]
Questionable carcinogen. | [Health Hazard]
Minimum toxic dose in humans is approximately 450 mg/kg (total dose) over 30 days for the ingested drug. Intravenous minimum toxic dose in humans is a total dose of 6 mg/kg over three days. Depression of white blood cells occurred after intravenous administrative of a total dose of 480 mg/kg over 32 days. Occasional neuropathy and cardiac toxicity have been reported. Do not use during pregnancy. Patients with impaired hepatic or renal function, with a history of high-dose pelvic irradiation or previous use of alkylating agents should be treated with extreme caution. Patients with nutritional deficiencies and protein depletion have a reduced tolerance to fluorouracil. | [Potential Exposure]
This material is used as an antineo plastic drug for cancer treatment and as a chemosterilant
for insects. | [Fire Hazard]
Emits very toxic fumes of flourides and nitrogen oxides when heated to decomposition. Avoid decomposing heat. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, includ ing resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medi cal attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit. Keep
victim quiet and maintain normal body temperature. | [Shipping]
UN2811 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name
Required. | [Incompatibilities]
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explo sions. Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides, methotrexrate sodium,
sources of heat. | [Description]
5-Fluorouracil (5-FU) is a prodrug form of the thymidylate synthase inhibitor fluorodeoxyuridylate (FdUMP).1 It is also converted to the active metabolites FUTP and FdUTP, which induce RNA and DNA damage, respectively. In vivo, 5-FU (15 mg/kg) when administered in combination with docetaxel (Item No. 11637) reduces tumor growth in B88 and CAL 27 oral squamous cell carcinoma (OSCC) mouse xenograft models.2 Formulations containing 5-FU have been used in the treatment of colorectal, breast, gastric, and pancreatic cancers. | [Chemical Properties]
Fluorouracil is a white crystalline solid.
Practically odorless. | [Chemical Properties]
White or almost white, crystalline powder | [Originator]
Efudex, Roche, US,1962 | [Definition]
ChEBI: 5-fluorouracil is a nucleobase analogue that is uracil in which the hydrogen at position 5 is replaced by fluorine. It is an antineoplastic agent which acts as an antimetabolite - following conversion to the active deoxynucleotide, it inhibits DNA synthesis (by blocking the conversion of deoxyuridylic acid to thymidylic acid by the cellular enzyme thymidylate synthetase) and so slows tumour growth. It has a role as a xenobiotic, an environmental contaminant, a radiosensitizing agent, an antineoplastic agent, an immunosuppressive agent and an antimetabolite. It is a nucleobase analogue and an organofluorine compound. It is functionally related to a uracil. | [Manufacturing Process]
A mixture of 200 grams (2 mols) of dry sodium fluoroacetate and 442 grams (2.86 mols) of diethyl sulfate was refluxed for 31? hours in an oil bath. The reaction mixture was then distilled through a fractionating column, yielding 177.3 grams of crude ethyl fluoroacetate, having a boiling range of 116° to 120°C. The material was redistilled through a fractionating column, yielding purified ethyl fluoroacetate boiling at 114° to 118°C.
In a 2-liter, 3-neck, round bottom flask, provided with stirrer, dropping funnel and reflux condenser, was placed 880 ml of absolute diethyl ether, and 47.6 grams (1.22 mols) of potassium, cut into 5 mm pieces, was suspended therein. 220 ml of absolute ethanol was added dropwise, while stirring, whereby the heat of reaction produced refluxing. In order to obtain complete dissolution of the potassium, the mixture was finally refluxed on a steam bath. The reaction mixture was then cooled in an ice bath, and a mixture of 135 grams (1.22 mols) of ethyl fluoroacetate and 96.4 grams (1.3 mols) of freshly distilled ethyl formate was added dropwise, while stirring and cooling, over a period of 2? hours. Upon completion of the addition of the ethyl formate, the reaction mixture was stirred for an additional hour while cooling, and then was allowed to stand overnight at room temperature.
At the end of this time the crystalline precipitate which had formed was filtered off with suction, washed with diethyl ether, and dried in a vacuum desiccator. The product comprised essentially the potassium enolate of ethyl fluoromalonaldehydate (alternative nomenclature, the potassium salt of fluoromalonaldehydic acid ethyl ester).
A mixture of 103.6 grams (0.6 mol) of the freshly prepared potassium enolate of ethyl fluoromalonaldehydate, 83.4 grams (0.3 mol) of Smethylisothiouronium sulfate and 32.5 grams (0.6 mol) of sodium methoxide was refluxed with stirring in 1,500 ml of absolute methanol. At first the reactants dissolved to a great extent, but very shortly thereafter precipitation occurred. The reaction mixture was refluxed for 2 hours and at the end of this time was evaporated to dryness in vacuo. The residue was treated with 280 ml of water; incomplete dissolution was observed.
The mixture obtained was clarified by filtering it through charcoal. The filtrate was acidified (to a slight Congo red acid reaction) by adding concentrated aqueous hydrochloric acid, containing 37% by weight HCl (48 ml required). The material which crystallized from the acidified solution was filtered off, washed free of sulfates with water and dried at 100°C, yielding crude Smethyl ether of 2-thio-5-fluorouracil, having a melting range from 202° to 221°C. The latter material was recrystallized by dissolving it in 2,035 ml of boiling ethylacetate and cooling to -20°C, yielding S-methyl ether of 2-thio-5fluorouracil, MP 230° to 237°C, in a sufficient state of purity that it could be used directly for the next step. A sample of the material was recrystallized from water (alternatively, from ethyl acetate) thereby raising the melting point to 241° to 243°C. For analysis the material was further purified by subliming it in vacuo at 140° to 150°/0.1 mm
A solution of 10.0 grams of purified S-methyl ether of 2-thio-5-fluorouracil, MP 230° to 237°C, in 150 ml of concentrated aqueous hydrochloric acid (containing approximately 37% by weight HCl) was refluxed under nitrogen for 4 hours. The reaction mixture was then evaporated in vacuo. The crystalline brownish residue was recrystallized from water. The resulting recrystallized product was further purified by sublimation in vacuo at 190° to 200°C (bath temperature)/0.1 mm pressure. There was obtained 5fluorouracil, in the form of colorless or pinkish-tan crystals, MP 282° to 283°C (with decomposition). | [Brand name]
Adrucil (Pharmacia & Upjohn); Adrucil (Sicor); Carac (Sanofi Aventis); Efudex (Valeant); Fluoroplex (Allergan). | [Therapeutic Function]
Cancer chemotherapy | [Synthesis Reference(s)]
Journal of Heterocyclic Chemistry, 20, p. 457, 1983 DOI: 10.1002/jhet.5570200236
Tetrahedron Letters, 21, p. 277, 1980 DOI: 10.1016/S0040-4039(00)71188-9 | [Biological Activity]
Anticancer agent. Metabolized to form fluorodeoxyuridine monophosphate (FdUMP), fluorodeoxyuridine triphosphate (FdUTP) and fluorouridine (FUTP). FdUMP inhibits thymidylate reductase causing a reduction in dTMP synthesis. FUTP and FdUTP are misincorporated into RNA and DNA respectively. | [Biochem/physiol Actions]
A potent antitumor agent that affects pyrimidine synthesis by inhibiting thymidylate synthetase, thus depleting intracellular dTTP pools. It is metabolized to ribonucleotides and deoxyribonucleotides, which can be incorporated into RNA and DNA. Treatment of cells with 5-FU leads to an accumulation of cells in S-phase and has been shown to induce p53 dependent apoptosis. | [Mechanism of action]
5-Fluorouracil (FU) is converted intracellularly to several active metabolites: fluorodeoxyuridine monophosphate (FdUMP), fluorodeoxyuridine triphosphate (FdUTP), and fluorouridine triphosphate (FUTP). The active metabolites of 5-FU disrupt RNA synthesis (FUTP), inhibit the action of thymidylate synthase (TS)—a nucleotide synthetic enzyme (FdUMP)—and can also be directly misincorporated into DNA (FdUTP). The rate-limiting enzyme in 5-FU catabolism is dihydropyrimidine dehydrogenase (DPD), which converts 5-FU to dihydrofluorouracil (DHFU). Over 80% of administered 5-FU is normally catabolized primarily in the liver, where DPD is abundantly expressed.
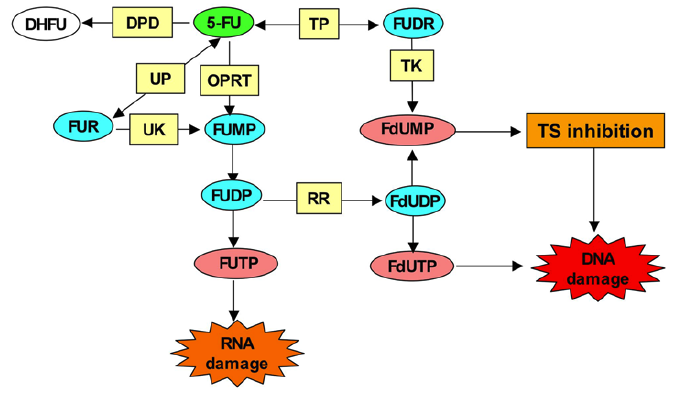
5-Fluorouracil (5-FU) is converted to three major active metabolites: (1) fluorodeoxyuridine monophosphate (FdUMP), (2) fluorodeoxyuridine triphosphate (FdUTP), and (3) fluorouridine triphosphate (FUTP). The main mechanism of 5-FU activation is conversion to fluorouridine monophosphate (FUMP) either directly by orotate phosphoribosyl transferase (OPRT), or indirectly via fluorouridine (FUR) through the sequential action of uridine phosphorylase (UP) and uridine kinase (UK). FUMP is then phosporylated to fluorouridine diphosphate (FUDP), which can be either further phosphorylated to the active metabolite fluorouridine triphosphate (FUTP), or converted to fluorodeoxyuridine diphosphate (FdUDP) by ribonucleotide reductase (RR). In turn, FdUDP can either be phosphorylated or dephosphorylated to generate the active metabolites FdUTP and FdUMP respectively. An alternative activation pathway involves the thymidine phosphorylase catalyzed conversion of 5-FU to fluorodeoxyuridine (FUDR), which is then phosphorylated by thymidine kinase (TK) to the thymidylate synthase (TS) inhibitor, FdUMP. Dihydropyrimidine dehydrogenase (DPD)-mediated conversion of 5-FU to dihydrofluorouracil (DHFU) is the rate-limiting step of 5-FU catabolism in normal and tumor cells. | [Mechanism of action]
Another action proposed for 5-fluorouracil may involve
the incorporation of the nucleotide 5-fluorouridine
triphosphate (5-FUTP) into RNA. The cytotoxic
role of these “fraudulent” 5-fluorouracil-containing
RNAs is not well understood.
Several possible mechanisms of resistance to 5-fluorouracil
have been identified, including increased synthesis
of the target enzyme, altered affinity of thymidylate
synthetase for FdUMP, depletion of enzymes
(especially uridine kinase) that activate 5-fluorouracil
to nucleotides, an increase in the pool of the normal
metabolite deoxyuridylic acid (dUMP), and an increase
in the rate of catabolism of 5-fluorouracil.
The drug has been administered orally, but absorption
by this route is erratic. The plasma half-life of 5-
fluorouracil after intravenous injection is 10 to 20 minutes.
It readily enters CSF. Less than 20% of the parent
compound is excreted into the urine, the rest being
largely metabolized in the liver. | [Pharmacology]
Local inflammatory reactions characterized
by erythema, edema, crusting, burning, and pain are
common (and, some would argue, desirable) but may be
minimized by reduced frequency of application or use
in combination with a topical corticosteroid. | [Clinical Use]
5-Fluorouracil (Efudex, Fluoroplex) is an antimetabolite
used for the topical treatment of actinic keratoses. It
is also useful for the treatment of superficial basal cell
carcinomas when conventional surgical modalities are
impractical. | [Clinical Use]
5-Fluorouracil (FU) is widely used in the treatment of a range of cancers including breast and cancers of the aerodigestive tract, but has had the greatest impact in colorectal cancer. 5-FU-based chemotherapy improves overall and disease-free survival of patients with resected stage III colorectal cancer. Nonetheless, response rates for 5-FU-based chemotherapy as a first-line treatment for advanced colorectal cancer are only between 10 and 15%. Combination of 5-FU with newer chemotherapies, such as irinotecan and oxaliplatin, has improved the response rates for advanced colorectal cancer to between 40 and 50%. | [Clinical Use]
5-Fluorouracil is used in several combination regimens
in the treatment of breast cancer. It also has palliative
activity in gastrointestinal adenocarcinomas, including
those originating in the stomach, pancreas, liver,
colon, and rectum. Other tumors in which some antitumor
effects have been reported include carcinomas of
the ovary, cervix, oropharynx, bladder, and prostate.
Topical 5-fluorouracil cream has been useful in the
treatment of premalignant keratoses of the skin and superficial
basal cell carcinomas, but it should not be used
in invasive skin cancer. | [Side effects]
Patients who are genetically deficient in this enzyme will experience a more pronounced effect from this drug and are at significant risk for use-limiting toxicity. In general, women clear fluorouracil faster than men do. Dosage adjustments usually are not required in hepatic or renal dysfunction. Major toxicities are related to bone marrow depression, stomatitis/esophagopharyngitis, and potential GI ulceration. Nausea and vomiting are common. Solutions of fluorouracil are light sensitive, but discolored products that have been properly stored and protected from light are still safe to use. | [Synthesis]
Fluorouracil, 4-fluorouracil (30.1.3.3), is made by condensing the ethyl ester
of fluoroacetic acid with ethylformate in the presence of potassium ethoxide, forming
hydroxy-methylenfluoroacetic ester (30.3.1), which cyclizes by reacting it with S-methyl�isothiourea to 2-methylthio-4-hydroxy-5-fluoropyrimidine, which is subsequently hydrolyzed
by hydrochloric acid to fluorouracil (30.1.3.3). An alternative method of synthesizing5-fluorouracid is direct fluorination of uracil with fluorine or trifluoromethylhypofluoride.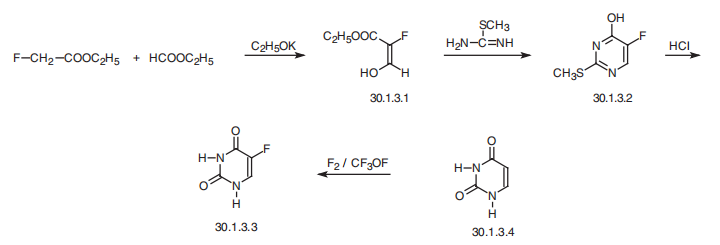
| [Veterinary Drugs and Treatments]
5-fluorouracil is a potent cytotoxic chemotherapeutic agent used
for the topical therapy of equine limbal and eyelid squamous cell
carcinoma. It is also used as an antimetabolite to limit fibrosis over
the body of gonioimplant devices used to artificially shunt aqueous
humor out of the eye in glaucoma as well as improve long-term
filtering performance of the implant.
1% solution applied to the affected eye three times daily. | [Drug interactions]
Potentially hazardous interactions with other drugs
Anticoagulants: possibly enhances effect of
coumarins.
Antipsychotics: avoid concomitant use with
clozapine, increased risk of agranulocytosis.
Cytotoxics: avoid with panitumumab.
Folic acid: toxicity of fluorouracil increased - avoid.
Metronidazole and cimetidine inhibit metabolism
(increased toxicity).
Temoporfin: increased skin photosensitivity with
topical fluorouracil | [Metabolism]
After intravenous injection fluorouracil is cleared rapidly
from plasma. It is distributed throughout body tissues
and fluids, and disappears from the plasma within about
3 hours. Within the target cell fluorouracil is converted
to 5-fluorouridine monophosphate and floxuridine
monophosphate (5-fluorodeoxyuridine monophosphate),
the former undergoing conversion to the triphosphate
which can be incorporated into RNA while the latter
inhibits thymidylate synthetase. About 15% of an
intravenous dose is excreted unchanged in the urine
within 6 hours. Approximately 80% is inactivated mainly
in the liver and is catabolised via dihydropyrimidine
dehydrogenase (DPD) similarly to endogenous uracil,
60-80% is excreted as respiratory carbon dioxide; urea
and other metabolites are also produced, and 2-3% by the
biliary system | [storage]
Store at +4°C | [References]
1) Schlisky (1998), Biochemical and Clinical Pharmacology of 5-Fluorouracil; Oncology, 12 13 |
| Questions And Answer | Back Directory | [Antimetabolite]
5-fluorouracil is short for fluorouracil, and is pyrimidine antimetabolites, 5-fluorouracil as fluorouracil for pyrimidine antimetabolites, is currently clinically commonly used a chemotherapy drug, having effect on proliferation, can prevent the thymine formation, inhibition of DNA biosynthesis, thereby inhibiting the growth of cancer cells. Clinically, it is used to treat gastrointestinal tumors, such as stomach cancer, colon cancer, liver cancer and so on. In breast cancer, ovarian cancer, lung cancer, bladder cancer, cervical cancer, pancreatic cancer and so on are also effective. The Swiss production of skin cancer treatment ointment containing 5% of the goods, mainly used for actinic keratoses and senile keratosis, precancerous dermatitis, single and multiple shallow table basal cell carcinoma, radioactive skin lesion of carcinoma and superficial basal cell carcinoma.
5-fluorouracil first changes for 5-Fluoro 2 deoxy urea pyrimidine nucleotides in vivo and inhibition of thymidylate synthase, blocking the transformation of urea pyrimidine deoxyribonucleotide thymidine, which affects DNA biosynthesis. At the same time, it can be incorporation into RNA by blocking urea ethyl pyridine and whey acid was incorporated into the RNA to direct inhibition of RNA synthesis.
This medicine is mainly in the liver metabolism, most of the decomposed into carbon dioxide discharged from breathing, rarely excreted from urine. After oral, absorption is different; vein after administration, concentrations in plasma quickly drop in two hours; static note within 30 minutes can arrive in cerebrospinal fluid (CSF) and maintain for 3 hours; continuous intravenous infusion toxicity is lighter than intravenous injection; vein to the drug's effect is compared with oral high. Toxicity of 5-fluorouracil on the proliferation is greater than non proliferating cells, but no obvious cell cycle specificity. Resistance to 5-FU can increase essential activity of enzyme missing or thymidylate synthetase activity.
The above information is edited by the Chemicalbook Hayan.
| [Pharmacokinetics]
Due to the instability of the absorption of 5-fluorouracil, the conventional the oral (in Europe can be obtained from oral preparation). General intravenous administration, We can also take transarterial Administration in order to directly reach the tumor (e.g. liver metastasis through hepatic artery) and injected directly into the body cavity infiltration liquid (such as ovarian cancer). Intravenous injection plasma half-life is 7.5~10 minutes, after 3 hours the drug in the plasma has not check did not change. Intracellular drug levels are last much longer.
Fluorouracil in the liver is used for metabolism; 60~80% in 8~12 hours as a respiratory carbon dioxide discharge and 15% in 6 hours technical unchanged from the urine discharge. The drug can enter into the exudate and cerebrospinal fluid (CSF). It has existed determination method for plasma fluorouracil.
| [Indications]
It is clinical for breast cancer, digestive tract cancer, ovarian cancer and primary bronchogenic lung adenocarcinoma adjuvant chemotherapy and palliative care; is also in the treatment of malignant hydatidiform mole, choriocarcinoma, serous cancer of effusion in bladder cancer and head and neck malignant tumor and liver cancer chemotherapy drugs.
Dermatological topical containing 5% 5-fluorouracil ointment is used in the treatment of actinic keratosis, actinic cheilitis, Bowen's disease, erythroplasia of Queyrat, Bowenoid papulosis, condyloma acuminatum, vitiligo, lichen amyloidosis, disseminated superficial porokeratosis, warts, flat warts, psoriasis, color of dry skin disease, superficial basal cell epithelioma table etc.; intralesional injection in the treatment of keratoacanthoma keloid.
| [Drug interaction]
Before using this drug, first it is used methotrexate, 5-fluorouracil nucleotide formation is increased by increasing the content of intracellular phosphoribosyl pyrophosphate. Allopurinol can change the role of fluorouracil. Its metabolites, oxypurinol, can inhibit orotate phosphoribosyl transferase and thus reduce the toxicity and may improve the therapeutic index. Increase in thymidine and other nucleoside combination of fluorouracil and RNA and thymidine by dihydropyrimidine dehydrogenase can delay fluorouracil decomposition. However, the drug combination did not significantly improve the clinical effect so far.
| [Adverse reactions and precautions]
The main toxic effect of fluorouracil is involving the gastrointestinal tract and blood cell generation system. Anorexia, nausea and vomiting were common. Stomatitis, pharyngo esophageal inflammation and diarrhea are withdrawal indication, otherwise there will be serious oropharyngeal and intestinal ulcers. Intravenous administration of gastrointestinal toxicity is often limiting dose. On the contrary, huge doses of intravenous injection, white cell reduction is the dose limiting toxicity. Low white cell counts often appear in medication for the first time after 7 to 14 days. Thrombocytopenia is not too obvious, appeared in 7~17. Monitoring of blood cell count is necessary.
Other adverse reactions are hair loss, dermatitis and pigment calm. There were acute and chronic conjunctivitis. Reversible cerebellar ataxia occurs in 1% of patients, possibly is related to the dose, occur at any time of the treatment process (often a few months later). After Cerebellar signs in the withdrawal can be last for a few of weeks. Myocardial ischemia occasionally appeared in the 5-FU intravenous drip. The drug in animals is caused by abnormal and may be carcinogenic.
Damage to the liver function of patients (e.g. extensive liver metastasis) fluorouracil should be reduced; The nutritional status of patients with poor medication should be cautious.
Using daily intermittent intravenous drip for 4~5d, can greatly reduce the toxic effects of blood. However, the results of clinical research mean rapid injection or intravenous drip method in the treatment of superiority. Long term intravenous drip infusion can be accompanied by pain, erythema and skin scaling of hand-foot comprehensive syndrome.
This medicine to FDA pregnancy category D.
| [Fluorofur]
Fluorofur is fluorine urea pyrimidine derivatives, and effect is similar with fluorouracil, but chemotherapy index double higher than fluorouracil and toxicity is only the 1/4 to 1/6 of fluorouracil. It is suitable for gastrointestinal cancer and breast cancer. There are oral, intravenous and anal suppository three formulations.
| [Chemical property]
It is white or white crystalline powder. Mp is 282-283℃ (decomposition), 0.1 mol/L hydrochloric acid solution has maximum absorption at 265nm wavelength. It is slightly soluble in water and ethanol, insoluble in chloroform and ether, soluble in dilute hydrochloric acid and sodium hydroxide solution. Medium toxicity, LD50 (mouse, i.p.) is 230mg/kg.
| [Uses]
1. It is used for biochemical studies and antitumor drugs.
2. It is the anti tumor drug, also used for synthesis of flucytosine. 5-fluorouracil can be used in the study of rice in the biochemical studies, ear differentiation, genetic metabolic measurement, plant growth development research.
3. It is used for the digestive system cancer, head and neck cancer, gynecological cancer, lung cancer, liver cancer, treatment of bladder cancer and skin cancer.
4. Antimetabolite antitumor drugs.
5. Anti tumor drugs. There is a certain effect on a variety of tumors such as digestive tract cancer, breast cancer, ovarian cancer, chorionic epithelial cancer, cervical cancer, hepatocellular carcinoma, bladder cancer, skin cancer (topical), leukoplakia (topical) etc. Adverse reactions mainly are bone marrow transplantation, digestive tract reaction, serious person can have diarrhea, local injection site phlebitis, a few of which have nervous system reactions such as cerebellar degeneration and ataxia. The course of medication should strictly check the blood.
| [Methods of production]
1. It is obtained by fluoride ethyl acetate by condensation, cyclization and hydrolysis.
(1). Condensation, cyclization. Sodium methoxide is input dry stainless steel reaction pot, stirring under vacuum concentration to sodium methoxide into white powder, cooling to 50℃, adding toluene, then cold to below 10℃, dropping ethyl formate. After adding remained below 10℃, dripping ethyl fluoroacetate. Completely, at about 30℃ stirring reaction for 8 hours. Static, obtain pale yellow thick mixture. In the condensation product, adding methanol and methyl isobutyl urea sulfate, stirring and heating to 66-70℃, reflux reaction for 6h. Atmospheric recovering methanol to the reaction material showing a thin paste, then vacuum distilled to viscous so far. Heating, dissolving in water, adding activated charcoal, filtered, and the filtrate with concentrated hydrochloric acid to pH3-4, crystallization, cooling and filtering, use cold water to wash the filter cake, using boiling water to regulate plasma immersion to recognize, filtering, water washing, drying, to 5-fluorouracil (-4-hydroxy-2-four oxygen pyrimidine C5H5FN2O2. (2). The hydrolysis of the cyclization product 5-Fluoro-4-hydroxy-2-methoxy pyrimidine and adding 20% hydrochloric acid in 60℃are hydrolysis for 4h, after processing to obtain 5-fluorouracil.
2. 2-methylthio-5-fluorouracil is under acidic conditions and reflux system to obtain 5-fluorouracil.
|
|
|