| Identification | Back Directory | [Name]
Alclometasone-17,21-dipropionate | [CAS]
66734-13-2 | [Synonyms]
Vitra
Almeta
Delonal
Ddlonal
Aclosone
Aclovate
sch22219
alclovate
Modrasone
AlcloMetasone-17
Ppropionic acid alclometasone
ALCLOMETASONEDIPROPIONATE,USP
(7-alpha,11-beta,16-alpha)-xy)
alclometasone-17,21-dipropionate
ALCLOMETHASONE-17,21-DIPROPIONATE
ALCLOMETASONE DIPROPIONATE (300 MG)
pregna-1,4-diene-3,20-dione,7-chloro-11-hydroxy-16-methyl-17,21-bis(1-oxopropo
7α-Chloro-11β-hydroxy-17,21-di(propionyloxy)-16α-methylpregna-1,4-diene-3,20-dione
7α-Chloro-16α-methyl-11β,17α,21-trihydroxypregna-1,4-diene-3,20-dione-17,21-dipropionate
(7a,11b,16a)-7-chloro-11-hydroxy-16-methyl-17,21-bis(1-oxopropoxy)pregna-1,4-diene-3,20-dione
Pregna-1,4-diene-3,20-dione,7-chloro-11-hydroxy-16-Methyl-17,21-bis(1-oxopropoxy)-, (7a,11b,16a)-
Pregna-1,4-diene-3,20-dione, 7-chloro-11-hydroxy-16-methyl-17,21-bis(1-oxopropoxy)-, (7α,11β,16α)-
7alpha-chloro-11beta,17,21-trihydroxy-16alpha-methylpregna-1,4-diene-3,20-dione 17,21-di(propionate) | [EINECS(EC#)]
266-464-3 | [Molecular Formula]
C28H37ClO7 | [MDL Number]
MFCD00865997 | [MOL File]
66734-13-2.mol | [Molecular Weight]
521.04 |
| Chemical Properties | Back Directory | [Description]
Alclometasone dipropionate is a potent topical steroid useful in the treatment of atopic dermatitis and psoriasis. | [Melting point ]
212-216° | [alpha ]
D26 +42.6° (c = 0.3 in DMF) | [Boiling point ]
613.3°C (rough estimate) | [density ]
1.0766 (rough estimate) | [refractive index ]
1.4429 (estimate) | [storage temp. ]
Hygroscopic, -20°C Freezer, Under inert atmosphere | [solubility ]
Chloroform (Slightly), DMF (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
13.70±0.70(Predicted) | [color ]
White to Off-White | [Stability:]
Hygroscopic |
| Hazard Information | Back Directory | [Usage]
Alclometasone-17,21-dipropionate is an synthetic glucocorticoid steroid used for inflammatory skin conditions. Alclometasone-17,21-dipropionate is topically used in dermatology as an anti-inflammator
y, antipruritic, antiallergic, antiproliferative and vasoconstrictive agent. | [Chemical Properties]
Alclometasone dipropionate is a white powder, insoluble in water, slightly soluble in propylene glycol and moderately soluble in hexylene glycol. Crystallization from acetone-methanol-isopropyl ether. | [Originator]
Schering (USA) | [Characteristics]
The alclometasone dipropionate [7α-chloro-11β,17,21- trihydroxy-16α-methylpregna-1,4-dien-3,20-dione 17,21- dipropionate) molecule is obtained by insertion of a chlorine atom in position 7α of 16α-me thylprensoline 17,21-dipropionate. The unique properties of the compound result from the presence of a chlorine atom in position 7α instead of positions C6 or C9, which increases the potency of its effect without increasing the incidence of local and systemic adverse effects. Additionally, as a highly lipophilic compound, alclometasone dipropionate rapidly penetrates into the skin where its active metabolites bind to specific receptors. | [Uses]
Alclometasone dipropionate is a corticosteroid used topically for its glucocorticoid activity in the treatment of various skin disorders. It is usually used as a cream or ointment containing 0.05%. It is topically used in dermatology as an anti-inflammatory, antipruritic, antiallergic, antiproliferative and vasoconstrictive agent. Alclometasone dipropionate has been used since 1986 for the treatment of corticosteroid-responsive dermatoses. It is ranked in Group 6 based on potency and has been dassified as group D. According to the A-D grouping, patch-test reactions occur six to seven times more frequently within well-defined groups of structurally related chemicals than between corticosteroids of different groups. | [Definition]
ChEBI: Alclometasone dipropionate is a prednisolone compound having an alpha-chloro substituent at the 7-position, an alpha-methyl substituent at the 16-position and O-propanoyl groups at the 17- and 21-positions. It has a role as an anti-inflammatory drug. It is a 20-oxo steroid, an 11beta-hydroxy steroid, a glucocorticoid, a steroid ester, a propanoate ester, a 3-oxo-Delta(1),Delta(4)-steroid and a chlorinated steroid. It is functionally related to a prednisolone. | [Preparation]
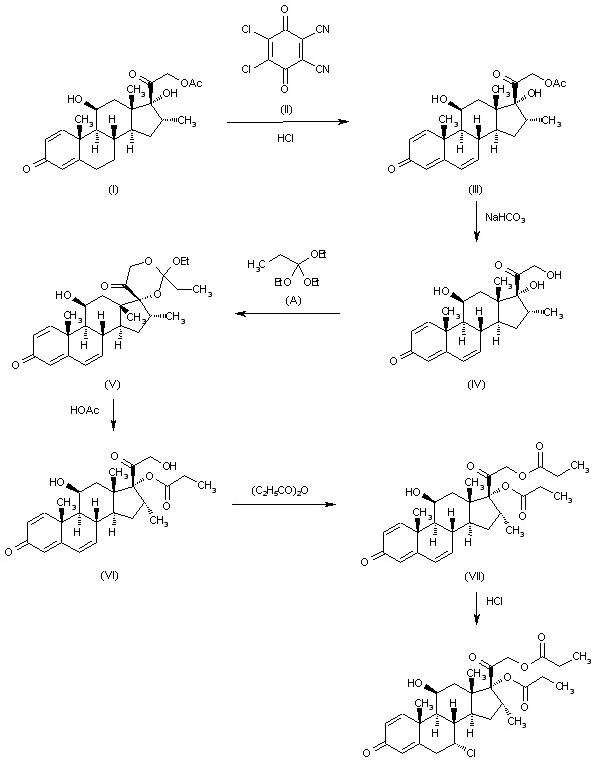
Alclometasone dipropionate synthesis: The dehydrogenation of 16alpha-methyl-11beta,17alpha,21-trihydroxypregna-1,4-diene-3,20-dione-21-acetate (I) with dichlorodicyanobenzoquinone (II) in dioxane HCl gives 16alpha-methyl-11beta,17alpha,21-trihydroxypregna-1,4,6-triene-3,20-dione-21-acetate (III), which is hydrolyzed with aqueous NaHCO3 to the corresponding free triol (IV). The reaction of (IV) with triethyl orthopropionate (A) by means of p-toluenesulfonic acid in DMSO yields 16alpha-methyl-11beta,17alpha,21-trihydroxypregna-1,4,6-triene-3,20-dione-17,21-ethylorthopropionate (V), which is hydrolyzed partially with acetic acid to 16alpha-methyl-11beta,17alpha,21-trihydroxypregna-1,4,6-triene-3,20-dione-17-propionate (VI). The acylation of (VI) with propionic anhydride affords 16alpha-methyl-11beta,17alpha,21-trihydroxypregna-1,4,6-triene-3,20-dione-17,21-dipropionate (VII), which is finally treated with dry HCl in dioxane.
 | [Manufacturing Process]
A). Add 16α-methyl-1,4,6-pregnatriene-11β,17α,21-triol-3,20-dione 17,21-
dipropionate (2.0 g) to dioxane (24 ml) which has been saturated with dry
hydrogen chloride gas. Stir at room temperature for 16 hours, pour into ice
water (600 ml), separate the resultant precipitate by filtration, wash the
precipitate with water and dry in air. Separate the components in the
foregoing precipitate on silica gel via thin layer chromatography utilizing as
developing solvent ether:hexane (2:1), and elute with ethyl acetate the band
containing 7α-chloro-16α-methyl-1,4-pregnadiene-11β,17α,21-triol-3,20-dione
17,21-dipropionate as shown by ultraviolet light. Evaporate the combined
ethyl acetate eluates and triturate the resultant residue with acetone:ether,
then filter and dry the triturated precipitate to obtain 7α-chloro-16α-methyl-
1,4-pregnadiene-11β,17α,21-triol-3,20-dione 17,21-dipropionate.
Alternatively, the 7α-chloro-16α-methyl-1,4-pregnadiene-17α,21-diol-3,11,20-
trione 17,21-dipropionate is prepared according to following procedures B and
C.
B). Saturate dry tetrahydrofuran (137 ml) at 0°C with dry hydrogen chloride
gas. Add 16α-methyl-1,4,6-pregnatriene-17α,21-diol-3,11,20-trione 17,21-
dipropionate (6.85 g) and stir the reaction mixture at 0°C for 1 hour. Pour into
ice water (1 liter) and stir for ? hour. Separate the resultant precipitate by
filtration, wash with water, and air dry to give 7v-chloro-16α-methyl-1,4-
pregnadiene-17α,21-diol-3,11,20-trione 17,21-dipropionate. Purify from
methanol:acetone containing a trace of propylene oxide; [α]D
26 +76.2°(dimethylformamide).
C). To a solution of 7v-chloro-16α-methyl-1,4-pregnadiene-17α,21-diol-
3,11,20-trione 17,21-dipropionate (3.2 g) in tetrahydrofuran (24 ml) and
methanol (8 ml) at 0°C under an atmosphere of nitrogen add sodium
borohydride (0.697 g) and stir the reaction mixture for 15 min at 0°C. Pour
into ice water (1.8 liters) and 250 ml of 1 N hydrochloric acid. Separate the
resultant precipitate by filtration and air dry to give 7α-chloro-16α-methyl-
11β,17α,21-triol-3,20-dione 17,21-dipropionate. Purify by crystallizing twice
from acetone:methanol:isopropyl ether; m.p. 212°-216°C; [α]D
26 +42.6°
(dimethylformamide). λmax 242 nm (methanol, ε 15,600).
By saponification of 7α-chloro-16α-methyl-1,4-pregnadiene-17α,21-diol-
3,11,20-trione 17,21-dipropionate is prepared (7α,11β,16α)-7-chloro-
11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione. | [Brand name]
Aclovate (GlaxoSmithKline), Aclosone, Legederm, Almeta, Vaderm, Modrasone, Delonal. | [Therapeutic Function]
Antiinflammatory, Antiallergic | [Side effects]
The following local adverse reactions have been reported with alclometasone dipropionate cream in approximately 2% of patients: itching and burning, erythema, dryness, irritation, and papular rashes.
The following local adverse reactions have been reported with alclometasone dipropionate ointment in approximately 1% of patients: itching, burning, and erythema. The following additional local adverse reactions have been reported infrequently with topical corticosteroids, but may occur more frequently with the use of occlusive dressings. These reactions are listed in approximate decreasing order of occurrence: folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, skin atrophy, striae, and miliaria. | [Safety Profile]
Alclometasone dipropionate has been classified as the FDA category C of drug safety during pregnancy (irrespective of the trimester of pregnancy), which means that it should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the foetus. The safety of alclometasone use in paediatric patients below 1 year of age has not been established and there are no adequate, randomised studies in large patient groups. | [storage]
Store at -20°C | [Mode of action]
At the cellular level, alclometasone dipropionate, like other glucocorticoids, after crossing the cell membrane binds to specific glucocorticoid receptors (GCR) in the cytoplasm. Subsequently, the glucocorticoid-GCR complex moves into the nucleus, where its binds to DNA at specific regions, known as the glucocorticoid response elements (GRE). At further stages, the expression of certain genes is either stimulated (transactivation) or inhibited (transuppression). In clinical practice, glucocorticoids are used for their anti-inflammatory, immunosuppressant and antiproliferative effects. |
|
| Company Name: |
LGM Pharma
|
| Tel: |
1-(800)-881-8210 |
| Website: |
www.lgmpharma.com |
| Company Name: |
Spectrum Chemical Manufacturing Corp.
|
| Tel: |
021-021-021-67601398-809-809-809 15221380277 |
| Website: |
www.spectrumchemical.com/oa_html/index.jsp?minisite=10020&respid=22372&language=us |
| Company Name: |
BOC Sciences
|
| Tel: |
1-631-485-4226; 16314854226 |
| Website: |
https://www.bocsci.com |
|