| Identification | More | [Name]
Hydroxyprogesterone | [CAS]
68-96-2 | [Synonyms]
17A-HYDROXY-4-PREGNENE-3,20-DIONE
17a-hydroxypregn-4-ene-3,20-dione
17A-HYDROXYPROGESTERONE
17ALPHA-HYDROXY-4-PREGNENE-3,20-DIONE
17-ALPHA-HYDROXYPREGN-4-ENE-3,20-DIONE
17ALPHA-HYDROXYPROGESTERONE
17-HYDROXYPROGESTERONE
4-PREGNEN-17ALPHA-OL-3,20-DIONE
4-pregnen-17a-ol-3,20-dione
4-PREGNEN-17-OL-3,20-DIONE
(8R,9S,10R,13S,14S,17R)-17-ACETYL-17-HYDROXY-10,13-DIMETHYL-1,2,6,7,8,9,10,11,12,13,14,15,16,17-TETRADECAHYDRO-CYCLOPENTA[A]PHENANTHREN-3-ONE
HYDROXYPROGESTERONE
HYDROXYPROGESTERONE, 17A-
17-Hydroxypregn-4-en-3,20-dione
17-hydroxy-pregn-4-ene-20-dione
17-hydroxypregn-4-ene-3,20-dione
delta(4)-pregnene-17alpha-ol-3,20-dione
gestagenogador
prodix
prodox | [EINECS(EC#)]
200-699-4 | [Molecular Formula]
C21H30O3 | [MDL Number]
MFCD00003659 | [Molecular Weight]
330.46 | [MOL File]
68-96-2.mol |
| Chemical Properties | Back Directory | [Appearance]
White Solid | [Melting point ]
276°C | [Boiling point ]
407.89°C (rough estimate) | [density ]
1.0998 (rough estimate) | [refractive index ]
90 ° (C=1, CHCl3) | [Fp ]
9℃ | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
soluble in Chloroform | [form ]
neat | [pka]
13.03±0.60(Predicted) | [color ]
White to Almost white | [Water Solubility ]
5.056mg/L(20 ºC) | [Usage]
Progesteron | [Merck ]
4839 | [BRN ]
3218109 | [CAS DataBase Reference]
68-96-2(CAS DataBase Reference) | [NIST Chemistry Reference]
Pregn-4-ene-3,20-dione, 17-hydroxy-(68-96-2) |
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
R61:May cause harm to the unborn child. | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S22:Do not breathe dust .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN1230 - class 3 - PG 2 - Methanol, solution | [WGK Germany ]
3
| [RTECS ]
TU5060000
| [HS Code ]
38220090 | [Hazardous Substances Data]
68-96-2(Hazardous Substances Data) |
| Hazard Information | Back Directory | [Chemical Properties]
White Solid | [Originator]
Prodox,Upjohn | [Uses]
17α-Hydroxy Progesterone is a metabolite of Progesterone. It was isolated from adrenal glands. | [Uses]
anticoagulant | [Uses]
Progesteron. It was isolated from adrenal glands. | [Definition]
ChEBI: A 17alpha-hydroxy steroid that is the 17alpha-hydroxy derivative of progesterone. | [Indications]
Hydroxyprogesterone has been used prophylactically
for the 12th to 37th week of pregnancy, particularly
in women who are in the high-risk category for premature
delivery (e.g., those with a history of premature delivery
or spontaneous abortion). A concern relating to
teratogenic potential has limited its use. Hydroxyprogesterone
as a tocolytic agent requires further evaluation
before its routine prophylactic administration
can be recommended. | [Manufacturing Process]
A suspension of 90.0 g of δ5-pregnen-3β,17α-diol-20-one in 2300 ml of 85%
formic acid was shaken for 2 h at a temperature of 70C. During this time the
compound partially dissolved and at the same time a new crystalline
substance appeared in the solution. After cooling, the precipitate was filtered,
thus giving 80.0 g of the 3-formate of δ5-pregnen-3β,17α-diol-20-one having
a melting point of 204°-207°C.
5.0 g of the 3-formate of δ5-pregnen-3β,17α-diol-20-one suspended in 120 ml
of acetic anhydride was treated with 1.5 g of p-toluenesulfonic acid and the
mixture was stirred for 9 h at room temperature. It was poured into water
and after 2 h standing, the precipitate was filtered and washed to neutral,
thus yielding the 3-formate 17-acetate of δ5-pregnen-3β,17α-diol-20-one in a
yield of over 90%.
1.0 g of 3-formate 17-acetate of δ5-pregnen-3β,17α-diol-20-one was dissolved
in 30 ml of xylene and 10 ml of cyclohexanone and 4 ml of the solution were
distilled in order to remove traces of moisture. 1.0 g of aluminum isopropylate
was added to the hot solution and the mixture was refluxed for 45 min. After
cooling to 90°C, water was added and the organic solvents were removed by
steam distiliation. Salt was added to the aqueous suspension and the residue
was filtered, dried and extracted with hot acetone. The acetone solution was
evaporated to dryness and the residue was crystallized from chloroform�methanol, thus giving 610.0 mg of the 17-acetate of δ4-pregnen-17α-ol-3,20-
dione (17-acetoxy-progesterone) with a melting point of 239°-240°C.
Saponification of this compound with 1% methanolic potassium hydroxide
yielded 80% of δ4-pregnen-17α-ol-3,20-dione. | [Therapeutic Function]
Progestin | [Biological Activity]
17-hydroxyprogesterone, an endogenous progestogen and chemical intermediate in the biosynthesis of other steroid hormones, is also the parent compound of various progestins derivatives. | [Synthesis]
Hydroxyprogesterone, 17|á-hydroxypregn-4-en-3,20-dione (28.3.6),
is synthesized from dehydropregnenolon (28.3.2). Dehydropregnenolon itself is made by
successive decomposition and oxidation of the side spiroketal group of diosgenin?athe aglycone
of one of the saponins of plant origin isolated from Discorea. The double bond at
C16¨CC17 or dehydropregnenolon is oxidized by hydrogen peroxide in the presence of a base
to give an epoxide (28.3.3). Interaction of the resulting epoxide with hydrogen bromide in
acetic acid forms a bromohydrin (28.3.4). The hydroxyl group of C3 of the steroid system is
formylated by formic acid, and reduction by hydrogen over a palladium catalyst removes the
bromine atom at C16, forming the product (28.3.5). The hydroxyl group at C17 of this product
is acylated by acetic acid anhydride and then the formyl group at C3 is oxidized by aluminum
isopropylate in the presence of cyclohexanone, during which simultaneous
isomerization takes place at the double bond, isomerizes from C5¨CC6 to position C4¨CC5,
forming the desired hydroprogesterone ester, in the given case an acetate (28.3.6), in which form it is used in medical practice. Other alternative ways of synthesis have been
proposed.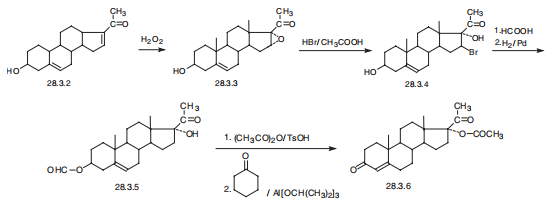
| [in vitro]
17-hydroxyprogesterone was found to be an agonist of the progesterone receptor (pr), which was similarly to progesterone. in addition, 17-hydroxyprogesterone was also an antagonist of the mineralocorticoid receptor (mr) as well as a partial agonist of the glucocorticoid receptor (gr), with very low potency (ec50>100-fold less relative to cortisol), which was also similarly to progesterone [1]. | [in vivo]
findings from a previous rat in vivo study demonstrated that even if modest, lowering blood pressure with 17-hydroxyprogesterone could be a viable treatment selection for blocking inflammation and uterine artery vasoconstriction, whereas improving litter size [2]. | [References]
[1] barbara j. attardi, et al. comparison of progesterone and glucocorticoid receptor binding and stimulation of gene expression by progesterone, 17-alpha hydroxyprogesterone caproate (17-ohpc), and related progestins. am j obstet gynecol. 2007 dec; 197(6): 599.e1–599.e7.
[2] lorena m. amaral, et al. 17- hydroxyprogesterone caproate significantly improves clinical characteristics of preeclampsia in the rupp rat model. hypertension. 2015 jan; 65(1): 225–231.
[3] ryckman kk,cook de,berberich sl,shchelochkov oa,berends sk,busch t,dagle jm,murray jc. replication of clinical associations with 17-hydroxyprogesterone in preterm newborns. j pediatr endocrinol metab. 2012;25(3-4):301-5. |
|
|