| Identification | More | [Name]
Progesterone | [CAS]
57-83-0 | [Synonyms]
4-PREGNEN-3,20-DIONE
4-PREGNENE-3,20-DIONE
(8S,9S,10R,13S,14S,17S)-17-ACETYL-10,13-DIMETHYL-1,2,6,7,8,9,10,11,12,13,14,15,16,17-TETRADECAHYDRO-CYCLOPENTA[A]PHENANTHREN-3-ONE
CORLUTIN
corporin
CYCLODEXTRIN-ENCAPSULATED PROGESTERONE
DELTA4-PREGNEN-3,20-DIONE
DELTA4-PREGNENE-3,20-DIONE
flavolutan
FOLOGENON
GESTONE
HOMOFLAVEINE
luteal hormone
LUTEOHORMONE
lutren
PRIMOLUT
PROGESTERONE
PROGESTERONE-WATER SOLUBLE
progestin
17alpha-Hydroxy-6alpha-methylpregn-4-ene-3,20-dione | [EINECS(EC#)]
200-350-6 | [Molecular Formula]
C21H30O2 | [MDL Number]
MFCD00003658 | [Molecular Weight]
314.46 | [MOL File]
57-83-0.mol |
| Chemical Properties | Back Directory | [Appearance]
Crystalline Powder | [Occurrence]
Colchicum luteum also yields this alkaloid. | [Melting point ]
128-132 °C (lit.) | [alpha ]
186 º (c=1, ethanol) | [Boiling point ]
394.13°C (rough estimate) | [density ]
d23 1.166; d20 1.171 | [refractive index ]
182 ° (C=2, Dioxane) | [Fp ]
2℃ | [storage temp. ]
room temp | [solubility ]
H2O: 25 mg/mL, may be clear to slightly hazy
| [form ]
powder
| [color ]
Yellow to yellow-brown | [Stability:]
Stable. Incompatible with strong oxidizing agents. | [biological source]
synthetic (organic) | [Water Solubility ]
<0.1 g/100 mL at 19 ºC | [Usage]
Sterid hormone produced by the corpus luteum. Induces maturation and secretory activity of the uterine endothelium; suppresses ovulation. Progesterone is implicated in the etiology of breast cancer | [Merck ]
7773 | [BRN ]
1915950 | [BCS Class]
4 | [Major Application]
pharmaceutical (small molecule) | [Cosmetics Ingredients Functions]
SKIN CONDITIONING | [InChI]
1S/C21H30O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h12,16-19H,4-11H2,1-3H3/t16-,17+,18-,19-,20-,21+/m0/s1 | [InChIKey]
RJKFOVLPORLFTN-LEKSSAKUSA-N | [SMILES]
CC(=O)[C@H]1CC[C@H]2[C@@H]3CCC4=CC(=O)CC[C@]4(C)[C@H]3CC[C@]12C | [LogP]
3.870 | [CAS DataBase Reference]
57-83-0(CAS DataBase Reference) | [NIST Chemistry Reference]
Progesterone(57-83-0) | [EPA Substance Registry System]
57-83-0(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,T | [Risk Statements ]
R40:Limited evidence of a carcinogenic effect.
R45:May cause cancer. | [Safety Statements ]
S36/37:Wear suitable protective clothing and gloves .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S53:Avoid exposure-obtain special instruction before use . | [RIDADR ]
UN 1648 3 / PGII | [WGK Germany ]
3
| [RTECS ]
TW0175000
| [TSCA ]
TSCA listed | [HS Code ]
29372300 | [Storage Class]
6.1C - Combustible acute toxic Cat.3
toxic compounds or compounds which causing chronic effects | [Hazard Classifications]
Carc. 2
Lact.
Repr. 1A | [Safety Profile]
NTP 10th Report
on Carcinogens. IARC Cancer Review:
Animal Lirmted Evidence IMEMDT 21,491,79; Animal Sufficient Evidence
IMEMDT 6,135,74. EPA Genetic
Toxicology Program. Reported in EPA
TSCA Inventory.
SAFETY PROFILE: Confirmed carcinogen
with experimental carcinogenic,
neoplastigenic, tumorigenic, and teratogenic
data. Poison by intravenous and
intraperitoneal routes. Human teratogenic
effects by ingestion and parenteral routes:
developmental abnormalities of the
urogenital system. Human male
reproductive effects by intramuscular route:
changes in spermatogenesis, the prostate,
seminal vesicle, Cowper's gland and
accessory glands, impotence, and breast
development. Human female reproductive
effects by ingestion, parenteral, and
intravaginal routes: ferthty changes;
menstrual cycle changes and disorders;
uterus, cervix, and vagina changes.
Experimental reproductive effects. Human
mutation data reported. When heated to
decomposition it emits acrid smoke and
irritating fumes. | [Hazardous Substances Data]
57-83-0(Hazardous Substances Data) | [Toxicity]
LD50 intraperitoneal in rat: 327mg/kg |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Cyclohexanone-->16-Dehydropregnenolone acetate-->Aluminium isopropoxide-->Pregnenolone | [Preparation Products]
Deoxycorticosterone acetate-->Dexamethasone-17-acetate-->17,21-dihydroxy-16beta-methylpregna-1,4,9(11)-triene-3,20-dione 21-acetate-->9-Bromo-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione-21-acetate-->9,11β-Epoxy-17-hydroxy-16β-methyl-3,20-dioxo-9β-pregna-1,4-diene-21-yl Acetate-->16,17-Epoxypregna-5,9(11)-diene-3,20-dione cyclic bis(1,2-ethanediyl acetal)-->11ALPHA-HYDROXYPROGESTERONE-->17-Hydroxy-16-methylpregna-5,9(11)-diene-3,20-dione 3,20-diethyleneketal-->PREGNANEDIONE-->16-Dehydroprogesterone-->METHYL 4-AZA-5ALPHA-ANDROSTA-3-ONE-17BETA-CARBOXYLATE-->Pregna-4,14-diene-3,20-dione-->5α-Pregnane-3,20-dione-->4-Aza-5a-androstan-1-ene-3-one-17b-carboxylic acid |
| Hazard Information | Back Directory | [General Description]
White powder. Melting point 121°C. Stable in air. Insoluble in water. A female sex hormone. Low toxicity. | [Reactivity Profile]
PROGESTERONE(57-83-0) is sensitive to light . | [Air & Water Reactions]
Insoluble in water. | [Hazard]
A carcinogen (OSHA).
| [Health Hazard]
ACUTE/CHRONIC HAZARDS: This chemical may be absorbed through the skin. | [Fire Hazard]
Flash point data for this compound are not available; however, PROGESTERONE is probably combustible. | [Chemical Properties]
White powder. Melting point 121°C. Stable in air. Insoluble in water. A female sex hormone. Low toxicity.
| [Definition]
ChEBI: A C21-steroid hormone in which a pregnane skeleton carries oxo substituents at positions 3 and 20 and is unsaturated at C(4)-C(5). As a hormone, it is involved in the female menstrual cycle, pregnancy and embryogenesis of humans and ot
er species. | [Definition]
progesterone: hormone, producedprimarily by the corpus luteumof the ovary but also by theplacenta, that prepares the inner liningof the uterus for implantation ofa fertilized egg cell. If implantationfails, the corpus luteum degeneratesand progesterone production ceasesaccordingly. If implantation occurs,the corpus luteum continues to secreteprogesterone, under the influenceof luteinizing hormone andprolactin, for several months of pregnancy,by which time the placentahas taken over this function. Duringpregnancy, progesterone maintainsthe constitution of the uterus andprevents further release of eggs fromthe ovary. Small amounts of progesteroneare produced by the testes. | [Brand name]
Crinone (Columbia); Prometrium (Unimed). | [Biological Functions]
Progesterone is a hormone, produced primarily by the corpus luteum of the ovary but also by the placenta, that prepares the inner lining of the uterus for implantation ofa fertilized egg cell. If implantation fails, the corpus luteum degenerates and progesterone production ceases accordingly. If implantation occurs,the corpus luteum continues to secrete progesterone, under the influence of luteinizing hormone and prolactin, for several months of pregnancy,by which time the placenta has taken over this function. Duringpregnancy, progesterone maintains the constitution of the uterus and prevents further release of eggs from the ovary. Small amounts of progesterone are produced by the testes. | [Biological Activity]
Endogenous progesterone receptor (PR) agonist (EC 50 = 0.5 nM). | [Biochem/physiol Actions]
Induces maturation and secretory activity of the uterine endothelium; suppresses ovulation. Progesterone is implicated in the etiology of breast cancer. | [Pharmacology]
During the second half of the menstrual cycle, progesterone promotes glandular growth in the endometrium, hyperemia of the uterus, thickening of the endometrium in preparation for implantation of a fertilized egg, and reduces the excitability of the uterus during pregnancy, inhibiting its activity and relaxing smooth muscles , allowing the embryo to grow safely. Under the joint action of estrogen, progesterone promotes the development of breast lobules and glands, so that the breasts can fully develop and prepare for lactation. Progesterone closes the cervix, reduces and thickens the mucus, and makes it difficult for sperm to penetrate; in large doses, it inhibits the secretion of pituitary gonadotropin through a negative feedback effect on the hypothalamus, resulting in the inhibition of ovulation. After ovulation, on the basis of the action of progesterone hormone, the endometrium continues to thicken and hyperemia, the glands proliferate and branch, from the proliferative phase to the secretory phase, which is conducive to the implantation and embryonic development of pregnant eggs. Progesterone inhibits uterine contractions and reduces the sensitivity of the uterus to oxytocin, allowing the fetus to grow safely. Progesterone competes against aldosterone, thereby promoting Na and Cl excretion and diuresis. Progesterone can slightly increase body temperature in normal women, so the basal body temperature in the luteal phase of the menstrual cycle is higher than that in the follicular phase. | [Side effects]
Common side effects of oral Progesterone include: chest pain, chills, cold or flu-like symptoms; cough or hoarseness; fever, and problems with urination. Rare side effects include: clear or bloody discharge; dimpling of the breast skin, lumps in the breast or armpit; persistent crusting or flaking; redness or swelling of the breast; and sores on the breast skin that do not heal. Other side effects that may occur are: abdominal or stomach pain; constipation, difficulty breathing, dizziness, fast, strong, or irregular heartbeat or pulse; headache, measles, indigestion, itching, joint pain, stiffness, or swelling; rash. | [Synthesis]
Progesterone, pregn-4-en-3,20-dione (28.3.1), is made by oxidizing pregnenolon
with aluminum isopropylate in the presence of cyclohexanone as a proton acceptor
(Oppenauer oxidation). Pregnenolon itself is made by subsequent oxidation
and further cleavage of the side chain of stigmasterin, a sterin of plant origin that is isolated
from soybeans.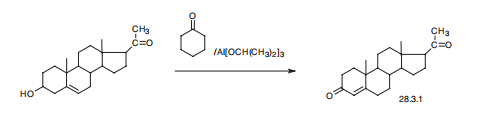
| [Carcinogenicity]
Progesterone is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals. | [storage]
Store at RT | [Purification Methods]
The ��form crystallises from EtOH with m 127-131o. The �-form crystallises from pet ether or aqueous pet ether/aqueous Et2O with m 119-120o or 121o. It also crystallises from Et2O, Me2CO/EtOAc, MeOH, aqueous Et2O, aqueous MeOH, wet pet ether, Et2O/pet ether, pet ether/*C6H6, Et2O/pentane and isopropyl ether. The is at 240nm with log 4.25 (EtOH). [Wintersteiner & Allen J Biol Chem 107 max 321 1934, Beilstein 7 III 3648, 7 IV 2395.] | [Toxics Screening Level]
The ITSL for progesterone is 0.1 μg/m3 based on an annual averaging time. | [References]
[1] BIJAN PEDRAM. A Tissue-Selective Nonsteroidal Progesterone Receptor Modulator: 7,9-Difluoro-5-(3-methylcyclohex-2-enyl)-2,2,4-trimethyl-1,2-dihydrochromeno[3,4-f]quinoline[J]. Journal of Medicinal Chemistry, 2008, 51 13: 3696-3699. DOI: 10.1021/jm8004256 |
| Questions And Answer | Back Directory | [Description]
Progesterone is a hormone released by luteal cells in the ovaries which contains 21 Carbon Atoms. Progesterone is also a crucial metabolic intermediate in the production of other endogenous steroids. There are two crystal forms of progesterone, that are type-α and type-β, the two types have similar physiological activity. Type-α is precipitated from dilute ethanol as orthorhombic white prismatic crystal, while type-β is orthorhombic white needle crystal, they are both insoluble in water, but soluble in ethanol, ethyl ether, chloroform, acetone, dioxane and concentrated sulfuric acid.
Progesterone can be released by the ovary, placenta and adrenal cortex. Its physiological function mainly manifests in promoting estrogen treated over thicken lining of the uterus to continue the development, proliferation and thickening and hypertrophy, soften and secretion of mucus in order to make good condition for implantation of the fertilized egg. After the implantation, early stage survival and development of fertilized egg is also under the control of the high progesterone release. As a result, progesterone is a very important hormone in the female reproductive system, and it is also an important intermediate in the biosynthesis of steroid hormones. All steroid hormone releasable glands can produce progesterone, but only ovarian and placenta can release progesterone as the main hormone. Ovarian will release large amount of progesterone in the luteal phase after ovulation by granulose luteal cells, so progesterone is also named as progestin. Progesterone will decrease in result of corpus luteum atrophy. For normal women, placenta will becomes the main organ maintain progesterone after 8 to 9 weeks of pregnancy, accompany with the ovary releasing until the end of pregnancy.
| [Uses]
The main physiological effects of progesterone:
1. Progesterone can maintain the female animal pregnancy, and cause a series of physiological changes, such as inhibition of female estrus.
2. Progesterone has the power to promote the thickening of the lining of the uterus, promote the bending of the gland and to increase secretion function.
3. Progesterone can inhibit the peristalsis of the uterus, and contribute to the cervix contraction, secretion of mucus, etc.. These physiological changes provide suitable environment for the operation, growth and development of early embryos, as well as the continued growth of the fetus.
4. Small amount of progesterone is also used in combination with the hormone estrogen to promote female estrus. The synergy between progesterone and prolactin can promote the development of mammary glands.
5. Progesterone is involved in the feedback regulation of the hypothalamus and anterior pituitary, which makes the balance of the animal reproductive hormones. In vivo, progesterone content of all sorts of livestock follicular phase is below 1 ng/ml, while in bovine corpus luteum period is approximately 4 ng/ml, pregnancy period is about 18 ng/ml.
6. Formerly biochemical study shows that progesterone modulates action as progestogens, clinical for the treatment of habitual abortion, dysmenorrhea, amenorrhea and other symptoms. One of progesterone's most important functions is as hormone drugs, to promote and maintain the uterine changes in the early stage of pregnancy, used in habitual abortion, irregular menstruation, etc.. In addition, progesterone also behaves as steroid hormone drug as well as progestogens, which is used in treatment of threatened abortion.
| [Preparation]
Progesterone can be obtained by oxidation of the pregnenolone. Dry toluene was added to a oven dried reaction kettle, and then cyclohexanone and pregnenolone were added in order with vigorous stirring to dissolve. Side product H2O was removed by Soxhlet extraction with toluene steam, aluminium isopropoxidequickly was added flowingly, the oxidation reaction was hold on at 115 oC for 2h, cooling to 80 oC, add 5% dilute sulfuric acid under stirring then stand by until water and toluene separated, the toluene layer was extracted with water to neutrality and then distillation off toluene and cyclohexanone. Cooling, filtering, filter cake was beated with petroleum, filtering, washing with petroleum, dried as crude progesterone. The crude product was dissolved in ethanol, decolorized by activated carbon, recrystallized to get the final product, yield 80%.
Another way to produce progesterone is choosing the 16-Dehydropregnenolone acetate as start material, treated consecutively by catalytic hydrogenation, alkali hydrolysis, oxidation by aluminum isopropoxide, to get the progesterone as final product.
|
|
|