| Identification | Back Directory | [Name]
Ethambutol | [CAS]
74-55-5 | [Synonyms]
emb
C06984
tibutol
MGC71745
diambutol
Etambutol
ETHAMBUTOL
d-ethambutol
EthaMbutol API
Ethambutol BP98
(+)-s,s-ethambutol
ETHAMBUTOL FREE BASE
Ethambutol USP/EP/BP
Acetylphenylacetylene
Anti-Embigin precursor
4-Phenyl-3-butyne-2-one
(3-Oxo-1-butynyl)benzene
Ethambutol HCL BP98,CP2000
Methyl phenylethynyl ketone
Phenylethynyl methyl ketone
ETHAMBUTOL HCL, USP STANDARD
2’-(ethylenediimino)di-(+)-1-butano
(+)-2,2-ethylenediiminodibutan-1-ol
d-2,2’-(ethylenediimino)di-1-butanol
Anti-EMB antibody produced in rabbit
(+)-2,2-(Ethylenediimino)di-1-butanol
(+)-2,2’-(ethylenediimino)di-1-butanol
d-2,2’-(ethylenediimino)bis(1-butanol)
2,2(1,2-Ethanediyldiimino)-bis-1-butanol
Ethambutol (base and/or unspecified salts)
2’-(1,2-ethanediyldiimino)bis-(r)-1-butano
2'-(1,2-ethanediyldiimino)bis-(r)-1-butanol
(2S,7S)-2,7-Diethyl-3,6-diazaoctane-1,8-diol
(r)-2,2’-(1,2-ethanediyldiimino)bis-1-butanol
(+)- (R,R)-NN'-Ethylene bis(2-aMinobutan-1-ol)
2,2'-(1,2-Ethanediyldiimino)bis[(2S)-1-butanol]
d,n,n’-bis(1-hydroxymethylpropyl)ethylenediamine
d-n,n’-bis(1-hydroxymethylpropyl)ethylenediamine
(+)-n,n’-bis(1-(hydroxymethyl)propyl)ethylenediamine
2,2’-(1,2-ethanediyldiimino)bis-,(r-(r*,r*))-1-butano
1-Butanol, 2,2'-(1,2-ethanediyldiimino)bis-, (2S,2'S)-
1-Butanol, 2,2'-(1,2-ethanediyldiimino)bis-, [S-(R*,R*)]-
(2S,2'S)-2,2'-(Ethane-1,2-diylbis(azanediyl))bis(butan-1-ol) | [EINECS(EC#)]
200-810-6 | [Molecular Formula]
C10H24N2O2 | [MDL Number]
MFCD00242828 | [MOL File]
74-55-5.mol | [Molecular Weight]
204.31 |
| Chemical Properties | Back Directory | [Melting point ]
199-204℃ | [alpha ]
D25 +13.7° (c = 2 in water) | [Boiling point ]
345℃ | [density ]
1.0048 (rough estimate) | [refractive index ]
1.4610 (estimate) | [RTECS ]
EL3640000 | [Fp ]
>110°(230°F) | [storage temp. ]
-20°C | [solubility ]
Soluble in DMSO | [form ]
Powder | [pka]
pKa 6.6 (H2O) (Uncertain);9.5(H3O) (Uncertain) | [color ]
White to off-white | [Water Solubility ]
Soluble in water. Also soluble in chloroform and methylene chloride | [InChI]
InChI=1S/C10H24N2O2/c1-3-9(7-13)11-5-6-12-10(4-2)8-14/h9-14H,3-8H2,1-2H3/t9-,10-/m0/s1 | [InChIKey]
AEUTYOVWOVBAKS-UWVGGRQHSA-N | [SMILES]
C(N[C@@H](CC)CO)CN[C@@H](CC)CO |
| Hazard Information | Back Directory | [Chemical Properties]
This product has a melting point of 87.5-88.8°C. It is soluble in chloroform, slightly soluble in benzene, and slightly soluble in water. Ethambutol hydrochloride is a white crystalline powder with a melting point of 198.5-200.3°C (201.8-202.6°C). It is readily soluble in water, slightly soluble in ethanol, and poorly soluble in acetone and chloroform. It is hygroscopic and has a slightly bitter taste. As a pharmaceutical, the dextrorotatory isomer is used clinically. | [Originator]
Myambutol,Lederle,US,1967 | [Uses]
Antibacterial. | [Definition]
ChEBI: An ethylenediamine derivative that is ethane-1,2-diamine in which one hydrogen attached to each of the nitrogens is sutstituted by a 1-hydroxybutan-2-yl group (S,S-configuration). It is a bacteriostatic antimycobacterial d
ug, effective against Mycobacterium tuberculosis and some other mycobacteria. It is used (as the dihydrochloride salt) in combination with other antituberculous drugs in the treatment of pulmonary and extrapulmonary tuberculosis; resistant str
ins of M. tuberculosis are readily produced if ethambutol is used alone. | [Indications]
Ethambutol is a water-soluble, heat-stable compound
that acts by inhibition of arabinosyl transferase enzymes
that are involved in cell wall biosynthesis. Nearly
all strains of M. tuberculosis and M. kansasii and most
strains of Mycobacterium avium-intracellulare are sensitive
to ethambutol. Drug resistance relates to point mutations
in the gene (EmbB) that encodes the arabinosyl
transferases that are involved in mycobacterial cell wall
synthesis. | [Manufacturing Process]
To 27 grams (2.55 mols) of 2-amino-1-butanol was added 100 grams (1.0
mol) of ethylene dichloride. The mixture was heated at reflux and in a few
minutes, the exothermic reaction required the removal of exterior heating.
After 10 minutes, exterior heating was recommenced for an additional 20
minutes. The hot mixture was then treated with 300 ml of methanol and then
cautiously with 84 grams (2.1 mols) of sodium hydroxide in 80 ml of water.
The precipitated sodium chloride was removed by filtration. The excess 2-
amino-1-butanol distilled as light yellow oil at 83° to 87°C/13 mm. The
viscous residue distilled at 165° to 170°C/0.6 mm as a light yellow oil which
tended to solidify in the air condenser; yield, 108 grams.
Recrystallization by dissolving in 80 ml of hot ethanol, adding about 150 ml of
petroleum ether (BP 90° to 100°C) and cooling at 5°C overnight, gave 64
grams of white crystals melting at 128° to 132.5°C. This, on recrystallization
from 100 ml of 95% ethanol, gave 35 grams of white crystals melting at
134.5° to 136°C and a second crop of 10 grams melting at 132.5° to 134°C
which is the meso base. Its dihydrochloride melts at 202° to 203°C.
From the ethanolic filtrates upon addition of 130 ml of about 4 N ethanolic
hydrochloric acid and cooling, there was obtained 55 grams of white crystals
melting at 176.5° to 178°C and a second crop of 10 grams melting at 171.5°
to 174.5°C. This is the dl racemate dihydrochloride. | [Brand name]
Myambutol (Stat Trade);Aethambutolum;Embutol;Etbutol. | [Therapeutic Function]
Antitubercular | [Antimicrobial activity]
Ethambutol is active against several species of mycobacteria
and nocardiae. MICs on solid media are: M. tuberculosis
0.5–2 mg/L; M. kansasii 1–4 mg/L; other slowly growing
mycobacteria 2–8 mg/L; rapidly growing pathogens 2–16
mg/L; Nocardia spp. 8–32 mg/L.
Resistance is uncommon and is a multistep process due to
mutations in the embA, embB and embC gene cluster. A mutation
in codon 306 of the embB gene predisposes to the development
of resistance to a range of antituberculosis agents,
possibly by affecting cell-wall permeability. | [Pharmaceutical Applications]
A synthetic ethylenediamine derivative formulated as the
dihydrochloride for oral administration. The dry powder is
very soluble and stable. | [Mechanism of action]
The mechanism of action of EMB remains unknown, although mounting evidence suggests a specific site of action for EMB. It has been known for some time that EMB affects mycobacterial cell wall synthesis; however, the complicated nature of the mycobacterial cell wall has made pinpointing the site of action difficult. In addition to the peptidoglycan portion of the cell wall, the mycobacterium have a unique outer envelop consisting of arabinofuranose and galactose (AG), which is covalently attached to the peptidoglycan and an intercalated framework of lipoarabinomannan (LAM) . The AG portion of the cell wall is highly branched and contains distinct segments of galactan and distinct segments of arabinan. At various locations within the arabinan segments (terminal and penultimate), the mycolic acids are attached to the C-5′ position of arabinan. Initially, Takayama et al. reported that EMB inhibited the synthesis of the AG portion of the cell wall. More recently, it has been reported that EMB inhibits the enzymes arabinosyl transferase. One action of arabinosyl transferase is to catalyze the polymerization of D-arabinofuranose, leading to AG. Ethambutol mimics arabinan, resulting in a buildup of the arabinan precursor β-D-arabinofuranosyl- 1-monophosphoryldecaprenol and, as a result, a block of the synthesis of both AG and LAM. The mechanism of resistance to EMB involves a gene overexpression o | [Pharmacokinetics]
Oral absorption: c. 80%, but some patients absorb it poorly
Cmax 25 mg/kg oral: 2–6 mg/L after 2–3 h
Plasma half-life: 10–15 h
Volume of distribution: >3 L/kg
Plasma protein binding: 20–30%
Absorption is impeded by aluminum hydroxide and alcohol.
It is concentrated in the phagolysosomes of alveolar macrophages.
It does not enter the cerebrospinal fluid (CSF) in
health but CSF levels of 25–40% of the plasma concentration,
with considerable variation between patients, are achieved in
cases of tuberculous meningitis.
Various metabolites are produced, including dialdehyde,
dicarboxylic acid and glucuronide derivatives. Around 50% is
excreted unchanged in the urine, with an additional 10–15%
as metabolites, and 20% is excreted unchanged in feces. | [Pharmacology]
Orally administered ethambutol is well absorbed
(70–80%) from the gut, and peak serum concentrations
are obtained within 2 to 4 hours of drug administration;
it has a half-life of 3 to 4 hours. Ethambutol is widely
distributed in all body fluids, including the cerebrospinal
fluid, even in the absence of inflammation.A
majority of the unchanged drug is excreted in the urine
within 24 hours of ingestion. Up to 15% is excreted in
the urine as an aldehyde and a dicarboxylic acid
metabolite. Ethambutol doses may have to be modified
in patients with renal failure. | [Clinical Use]
Ethambutol has replaced aminosalicylic acid as a
first-line antitubercular drug. It is commonly included as
a fourth drug, along with isoniazid, pyrazinamide, and
rifampin, in patients infected with MDR strains. It also
is used in combination in the treatment of M. aviumintracellulare
infection in AIDS patients. | [Clinical Use]
Tuberculosis (initial intensive phase of short-course therapy)
Other mycobacterioses (M. kansasii, M. xenopi, M. malmoense and the
M. avium complex) (with appropriate additional drugs) | [Side effects]
The major toxicity associated with ethambutol use is
retrobulbar neuritis impairing visual acuity and redgreen
color discrimination; this side effect is dose related
and reverses slowly once the drug is discontinued.
Mild GI intolerance, allergic reaction, fever, dizziness,
and mental confusion are also possible. Hyperuricemia
is associated with ethambutol use due to a decreased renal
excretion of urates; gouty arthritis may result. | [Side effects]
The most important side effect is optic neuritis, which may be
irreversible if treatment is not discontinued. This complication
is rare if the higher dose (25 mg/kg) is given for no more
than 2 months. National codes of practice for prevention of
ocular toxicity should be adhered to; in particular, patients
should be advised to stop therapy and seek medical advice if
they notice any change in visual acuity, peripheral vision or
color perception, and the drug should not be given to young
children and others unable to comply with this advice.
Other side effects include gastrointestinal upsets, peripheral
neuritis, arthralgia, nephritis, myocarditis, hyperuricemia,
dermal hypersensitivity and, rarely, thrombocytopenia
and hepatotoxicity. | [Synthesis]
Ethambutol, (±)-N,N�-ethylenbis-(2-aminobutan-1-ol) (34.1.4), is synthe�sized in several different ways. According to one of them, nitropropane undergoes oxymethylation using formaldehyde, and the nitro group in the resulting 2-nitrobutanol (34.1.2) is reduced by hydrogen to an amino group, making racemic (±) 2-aminobutanol. L (£?) tartaric acid is used to separate (£?) 2-aminobutanol (34.1.3). Reacting this with 1, 2-dichloroethane in the presence of sodium hydroxide gives ethambutol (34.1.4).
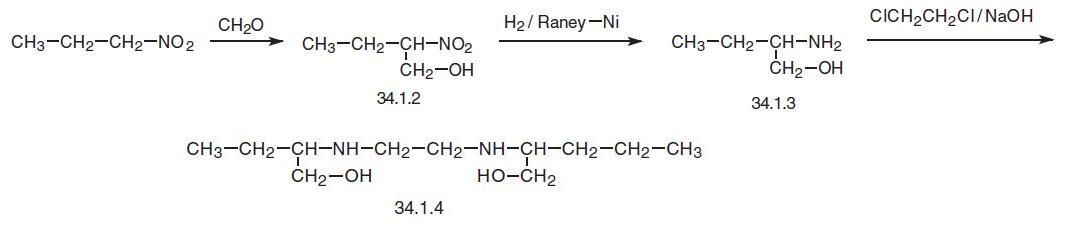
An alternative method of synthesis consists of preparing (£?) 2-aminobutanol (34.1.3) by reducing ethyl ester of L-2-aminobutyric acid hydrochloride with hydrogen using simulta�neously Raney nickel and platinum oxide catalysts. This gives pure (£?) 2-aminobutanol. Reacting this with 1,2-dichloroethane in the presence of sodium hydroxide gives the desired ethambutol (34.1.4).

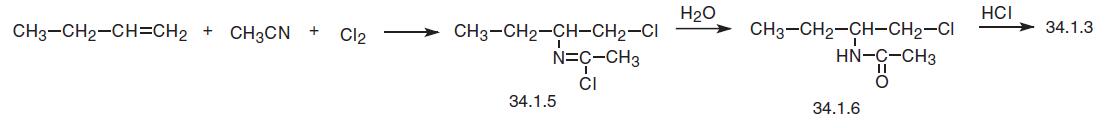
The third way of synthesis is very interesting and resembles of the Ritter reaction, but which takes place in the presence of chlorine. This method consists of reacting 1-butene and acetonitrile in the presence of chlorine, which evidently results in the 1,4-addition of chlorine to the product of the Ritter reaction, forming an intermediate dichloride (33.1.5), which is hydrolyzed with water to make N-[1-(chloromethyl)-propyl]-acetamide (33.1.6). Heating this product with hydrochloric acid gives racemic (±) 2-aminobutanol, from which (£?) 2-aminobutanol (34.1.3) is isolated as described above using L (£?) tartaric acid. Reacting this with 1,2-dichloroethane in the presence of sodium hydroxide gives the desired ethambutol (34.1.4)
| [storage]
Store at -20°C |
|
|