| Identification | More | [Name]
Pyrazinamide | [CAS]
98-96-4 | [Synonyms]
2-PYRAZINECARBOXAMIDE
AKOS NCG1-0042
LABOTEST-BB LT00233122
PYRAZINAMIDE
PYRAZINE-2-CARBOXAMIDE
PYRAZINE-2-CARBOXYLIC ACID AMIDE
PYRAZINECARBOXAMIDE
PYRAZINOIC ACID AMIDE
PZAD
TIMTEC-BB SBB004276
2-Carbamylpyrazine
Aldinamid
Aldinamide
Eprazin
Farmizina
MK 56
mk56
NCI-C01785
Novamid
Pirazimida | [EINECS(EC#)]
202-717-6 | [Molecular Formula]
C5H5N3O | [MDL Number]
MFCD00006132 | [Molecular Weight]
123.11 | [MOL File]
98-96-4.mol |
| Chemical Properties | Back Directory | [Appearance]
Crystalline Solid | [Melting point ]
189-191 °C (lit.) | [Boiling point ]
229.19°C (rough estimate) | [density ]
1.3260 (rough estimate) | [refractive index ]
1.5900 (estimate) | [Fp ]
>110°(230°F) | [storage temp. ]
2-8°C | [solubility ]
H2O: soluble50mg/mL | [form ]
Crystalline Powder or Needles | [pka]
0.5(at 25℃) | [color ]
White | [PH]
7 (H2O) | [Water Solubility ]
15 mg/mL | [Usage]
Antibacterial (tuberculostatic) | [Merck ]
7956 | [BRN ]
112306 | [BCS Class]
1,3 | [Major Application]
pharmaceutical (small molecule) | [InChI]
1S/C5H5N3O/c6-5(9)4-3-7-1-2-8-4/h1-3H,(H2,6,9) | [InChIKey]
IPEHBUMCGVEMRF-UHFFFAOYSA-N | [SMILES]
NC(=O)c1cnccn1 | [LogP]
-0.600 | [CAS DataBase Reference]
98-96-4(CAS DataBase Reference) | [NIST Chemistry Reference]
Pyrazine carboxamide(98-96-4) | [EPA Substance Registry System]
98-96-4(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
F,C | [Risk Statements ]
R11:Highly Flammable.
R34:Causes burns. | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S16:Keep away from sources of ignition-No smoking . | [WGK Germany ]
3
| [RTECS ]
UQ2275000
| [TSCA ]
Yes | [HS Code ]
29339990 | [Storage Class]
11 - Combustible Solids | [Hazardous Substances Data]
98-96-4(Hazardous Substances Data) | [Toxicity]
LD50 intraperitoneal in mouse: 1680mg/kg |
| Hazard Information | Back Directory | [General Description]
White powder. Sublimes from 318°F. | [Reactivity Profile]
PYRAZINAMIDE(98-96-4) is a carbamate ester. Incompatible with strong acids and bases, and especially incompatible with strong reducing agents such as hydrides. May react with active metals or nitrides to produce flammable gaseous hydrogen. Incompatible with strongly oxidizing acids, peroxides, and hydroperoxides. | [Air & Water Reactions]
Water soluble. | [Description]
Pyrazinamide was synthesized in 1952, and it is the nitrogen-analog of nicotinamide. It
exhibits hepatotoxicity. Synonyms of this drug are dexambutol, miambutol, esnbutol, ebu�tol, and others. | [Chemical Properties]
Crystalline Solid | [Definition]
ChEBI: Pyrazinecarboxamide is a monocarboxylic acid amide resulting from the formal condensation of the carboxy group of pyrazinoic acid (pyrazine-2-carboxylic acid) with ammonia. A prodrug for pyrazinoic acid, pyrazinecarboxamide is used as part of multidrug regimens for the treatment of tuberculosis. It has a role as an antitubercular agent and a prodrug. It is a member of pyrazines, a N-acylammonia and a monocarboxylic acid amide. | [Antimicrobial activity]
It is principally active against actively metabolizing intracellular
bacilli and those in acidic, anoxic inflammatory lesions.
Activity against M. tuberculosis is highly pH dependent: at pH
5.6 the MIC is 8–16 mg/L, but it is almost inactive at neutral
pH. Other mycobacterial species, including M. bovis, are resistant.
Activity requires conversion to pyrazinoic acid by the
mycobacterial enzyme pyrazinamidase, encoded for by the
pncA gene, which is present in M. tuberculosis but not M. bovis.
A few resistant strains lack mutations in pncA, indicating alternative
mechanisms for resistance, including defects in transportation
of the agent into the bacterial cell. | [Acquired resistance]
Drug resistance is uncommon and cross-resistance to other
antituberculosis agents does not occur. Susceptibility testing
is technically demanding as it requires very careful control of
the pH of the medium, but molecular methods for detection
of resistance-conferring mutations are available. | [Pharmaceutical Applications]
Like isoniazid, pyrazinamide is a synthetic nicotinamide analog,
although its mode of action is quite distinct. | [Biochem/physiol Actions]
The active moiety of pyrazinamide is pyrazinoic acid (POA). POA is thought to disrupt membrane energetics and inhibit membrane transport function at acid pH in Mycobacterium tuberculosis. Iron enhances the antituberculous activity of pyrazinamide . Pyrazinamide and its analogs have been shown to inhibit the activity of purified FAS I. | [Pharmacokinetics]
Oral absorption: >90%
Cmax 20–22 mg/kg oral: 10–50 mg/L after 2 h
Plasma half-life: c. 9 h
Plasma protein binding: c. 50%
It readily crosses the blood–brain barrier, achieving CSF
concentrations similar to plasma levels. It is metabolized to
pyrazinoic acid in the liver and oxidized to inactive metabolites,
which are excreted in the urine, although about 70% of
an oral dose is excreted unchanged. | [Pharmacology]
Pyrazinamide is well absorbed from the GI tract and
is widely distributed throughout the body. It penetrates
tissues, macrophages, and tuberculous cavities and has
excellent activity on the intracellular organisms; its
plasma half-life is 9 to 10 hours in patients with normal
renal function. The drug and its metabolites are excreted
primarily by renal glomerular filtration. | [Clinical Use]
Pyrazinamide is an essential component of the multidrug
short-term therapy of tuberculosis. In combination
with isoniazid and rifampin, it is active against the
intracellular organisms that may cause relapse. | [Clinical Use]
Tuberculosis (a component of the early, intensive phase of short-course
therapy) | [Side effects]
Hepatotoxicity is the major concern in 15% of pyrazinamide
recipients. It also can inhibit excretion of urates,
resulting in hyperuricemia. Nearly all patients taking
pyrazinamide develop hyperuricemia and possibly acute
gouty arthritis. Other adverse effects include nausea,
vomiting, anorexia, drug fever, and malaise. Pyrazinamide
is not recommended for use during pregnancy. | [Side effects]
It is usually well tolerated. Moderate elevations of serum
transaminases occur early in treatment. Severe hepatotoxicity
is uncommon with standard dosage, except in patients with
pre-existing liver disease.
Its principal metabolite, pyrazinoic acid, inhibits renal
excretion of uric acid, but gout is extremely rare. An unrelated
arthralgia, notably of the shoulders and responsive to
analgesics, also occurs.
Other side effects include anorexia, nausea, mild flushing
of the skin and photosensitization. | [Synthesis]
Pyrazinamide, pyrazincarboxamide (34.1.11), is synthesized from quinox�aline (34.1.7) by reacting o-phenylendiamine with glyoxal. Oxidation of this compound
with sodium permanganate gives pyrazin-2,3-dicarboxylic acid (34.1.8). Decarboxylation
of the resulting product by heating gives pyrazin-2-carboxylic acid (34.1.9). Esterifying
the resulting acid with methanol in the presence of hydrogen chloride and further reaction
of this ester (34.1.10) with ammonia gives pyrazinamide.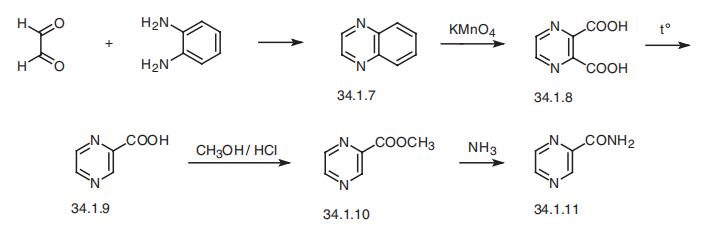
Pyrazinamide was synthesized in 1952, and it is the nitrogen-analog of nicotinamide. It
exhibits hepatotoxicity. Synonyms of this drug are dexambutol, miambutol, esnbutol, ebu�tol, and others. | [Metabolism]
Pyrazinamide is metabolised mainly in the liver by
hydrolysis to the major active metabolite pyrazinoic acid,
which is subsequently hydroxylated to the major excretory
product 5-hydroxypyrazinoic acid.
It is excreted via the kidneys mainly by glomerular
filtration. About 70% of a dose appears in the urine
within 24 hours mainly as metabolites. | [Purification Methods]
The amide crystallises from water, EtOH or 1:1 hexane/EtOH in four modifications viz �-form, �-form, -form and ��form. [R. & S.rum Acta Cryst 28B 1677 1972, Beilstein 25 III/IV 772.] | [References]
[1] Tetrahedron Letters, 2017, vol. 58, # 50, p. 4709 - 4712
[2] Tetrahedron, 1985, vol. 41, # 19, p. 4157 - 4170
[3] Chemical Communications, 2002, # 21, p. 2496 - 2497 |
| Questions And Answer | Back Directory | [Anti-tuberculosis drug]
Pyrazinamide is a second-line anti-tuberculosis drug, also known as formamide pyrazine, carbamoyl pyrazine, and isonicotinic acid amine. At room temperature, it appears as a white crystalline powder and is slightly soluble in water and is odorless with slightly bitter taste. It has a good antibacterial effect against human type Mycobacterium tuberculosis with the strongest bactericidal effect at the range of pH value being between 5-5.5. It has especially optimal bactericidal effect against the Mycobacterium tuberculosis inside the slow-growing phagocytic cells in acidic environment. After pyrazinamide penetrates into the phagocytic cells and enter into the body of Mycobacterium tuberculosis, lactamase in vivo make it be de-amidated, being converted to pyrazine acid to play the antibacterial effect.
The in vivo inhibitory concentration is 12.5μg/ml with the concentration of 50 μg/ml being able to kill the Mycobacterium tuberculosis. The inhibitory concentration against Mycobacterium tuberculosis in vivo is 10 times lower than that in vitro with almost no inhibitory effect in a neutral, alkaline environment.
Its anti-bacterial effect is between streptomycin and paramisansodium. It has great toxicity and can easy to produce drug resistance and should be used in combination with other anti-TB drugs.
Pyrazinamide has similar chemical structure with nicotinamide and can interfere with the dehydrogenase through substitution of nicotinamide, therefore preventing the dehydrogenation and inhibiting the utilization of oxygen by Mycobacterium tuberculosis, causing death of the bacteria due to failure of normal metabolism.
It is oral easily absorbed and is widely distributed in body tissues and fluids including liver, lung, cerebrospinal fluid, kidney and bile. After 2 hours, its plasma concentration can reach peak. The concentration of cerebrospinal fluid is similar as blood concentrations. It can subject to hepatic metabolism to be hydrolyzed to the pyrazine acid that is a kind of metabolite having antimicrobial activity, then further being hydroxylated into inactive metabolites and excreted in urine after glomerular filtration. The t1/2 is about 8 to 10 hours. It can be used in combination with other kind of anti-TB drugs fro the treatment of some complex cases of tuberculosis and tubercular meningitis patients.
| [Drug Interactions]
1, when being combined with allopurinol, colchicine, probenecid, and sulfinpyrazone, pyrazinamide can increase the serum uric acid concentration and further reduce the efficacy of the above drugs on gout. Therefore, when being combined with pyrazinamide, the above drugs should be subject to dose adjustment in order to control hyperuricemia and gout.
2, it can enhance the adverse reactions when combined with B sulfur isonicotinoyl amine.
3, when cyclosporine is used simultaneously with pyrazinamide, the blood concentration of the former drug may be reduced, and therefore the blood concentration needs to be monitored and we should adjust the dose if necessary.
4, it has synergistic effect when combined with isoniazid and rifampin and can delay the development of drug resistance.
The above information is edited by the chemicalbook of Dai Xiongfeng.
| [Indications]
It can be used in combination with other anti-TB drugs for the treatment of tuberculosis that failed to be cured by first-line anti-TB drugs (such as streptomycin, isoniazid, rifampicin and ethambutol).
This product is only valid against mycobacteria.
In the past, pyrazinamide was used as second-line drugs, commonly applied to the patients undergoing retirement due to failure to be cured by other anti-TB drugs. A large number of clinical studies have shown: the short course regimen containing this product is suitable for being applied to the newly diagnosed sputum-positive cases. It is generally applied for 2 to 3 months. This protocol can enable a significant reduction of the re-positive rate of Mycobacterium tuberculosis after the end of treatment.
This product has been well considered as the composition of triple or quadruple protocols in short course chemotherapy.
| [Dosage]
When used in combination therapy with other anti-TB drugs, the common dose of adult oral administration is: every 6 hours according to the weight 5-8.75mg/kg, or every eight hours according to the weight 6.7-11.7mg/kg; the highest value is 3 g daily.
Upon treatment of the infection of isoniazid resistant bacteria, you can increase the dose to 60 mg/kg daily.
Children should take with caution, the necessary reference amount should be: 20-25mg/kg daily, it should be separately orally administrated in 3 times with the maximum dose being 2 g daily, the treatment course is generally 2 to 3 months, it can not be more than six months.
| [First aid treatment]
1. Misusage patients should be immediately subject to gastric lavage and catharsis.
2. If liver dysfunction occurs during the course of treatment, the drug should be discontinued and routine liver-protection therapy should be applied.
3. Patients of gout should be given 0.25g/time probenecid (carboxymethyl benzene with oral administration in 3 times daily and being able to promote the excretion of uric acid.
4. Allergic patients should be given treatment with antihistamines and corticosteroids.
| [Adverse reactions and side effects]
Long-term or high-dose application of the product is easy to cause liver damage and increased blood uric acid and can also cause gastrointestinal irritation and allergic reactions.
For patients of relative high incidence: loss of appetite, fever, unusual fatigue or weakness, yellowing of the eyes or skin (liver toxicity).
Persons of low incidence: chills, joint pain (especially in the big toe, the condyle, knee) or diseased joints skin taut fever (acute gouty joint pain).
During the treatment course of this drug, the blood uric acid can increase and can cause acute gout that should be subject to determination of serum uric acid.
Adverse reactions are dose-related. After current application of conventional dosage, adverse reactions have been rarely observed.
Hepatic impairment: administration of drug for 3g daily with about 15% of patients getting liver damage, hepatomegaly, tenderness, elevated transaminases and jaundice. Currently upon applying 1.5 g daily for a 3-month treatment course, reactions of liver toxicity are rare.
Joint pain: PZA metabolites can inhibit the excretion of uric acid, causing hyperuricemia and gout-like performance with resumption after stopping drug.
Gastrointestinal reactions: loss of appetite, nausea, vomiting.
Allergies: occasionally fever and rash, and even jaundice.
Skin reactions: in some individual cases, the patients are light sensitive with the exposed parts of the skin being bright red brown. Patients subject to the long-term medication have their skins be bronze that can be gradually restored after the withdrawal of the drug.
For diabetic patients taking pyrazinamide, it is difficult to control the level of blood sugar.
| [Uses]
It is a kind of anti-tuberculosis drugs.
|
|
|