| Identification | Back Directory | [Name]
Isoproterenol | [CAS]
7683-59-2 | [Synonyms]
A 21
Aludrin
isoprel
asmalar
NSC 9975
Aludrine
Aleudrine
ICI 46399
NSC 33791
Respifral
asiprenol
Vapo-N-Iso
assiprenol
saventrine
bellasthman
dl-Isadrine
isoprenaline
isoproterenol
dl-Isoprenaline
Racemic isoprenaline
Racemic isoproterenol
Isoproterenol USP/EP/BP
dl-Isopropylnoradrenaline
DL-Isopropylnorepinephrine
dl-N-Isopropylnoradrenaline
Epinephrine isopropyl homolog
dihydroxyphenylethanolisopropylamine
Isoproterenol Isoproterenol Hydrochloride
Isoproterenol (base and/or unspecified salts)
1-(3,4-dihydroxyphenyl)-2-isopropylaminoethanol
alpha-(isopropylaminomethyl)protocatechuyl alcohol
4-(2-Isopropylamino-1-hydroxyethyl)-1,2-benzenediol
4-[1-Hydroxy-2-(isopropylamino)ethyl]-1,2-benzenediol
4-(1-hydroxy-2-(isopropylaMino)ethyl)benzene-1,2-diol
.alpha.-(Isopropylaminomoethyl)protocatechuyl alcohol
4-[1-hydroxy-2-(propan-2-ylamino)ethyl]benzene-1,2-diol
1,2-Benzenediol, 4-1-hydroxy-2-(1-methylethyl)aminoethyl-
3,4-dihydroxy-alpha-((isopropylamino)methyl)benzyl alcohol
(RS)-4-[1-hydroxy-2-(isopropylamino)ethyl]benzene-1,2-diol
4-(1-hydroxy-2-((1-methylethyl)amino)ethyl)-1,2-benzenediol
1,2-Benzenediol, 4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]- (9CI) | [EINECS(EC#)]
231-687-7 | [Molecular Formula]
C11H17NO3 | [MOL File]
7683-59-2.mol | [Molecular Weight]
211.26 |
| Hazard Information | Back Directory | [Uses]
Bronchodilator. | [Definition]
ChEBI: A secondary amino compound that is noradrenaline in which one of the hydrogens attached to the nitrogen is replaced by an isopropyl group. A sympathomimetic acting almost exclusively on beta-adrenergic receptors, it is used (mainly as the hy
rochloride salt) as a bronghodilator and heart stimulant for the management of a variety of cardiac disorders. | [Brand name]
Medihaler-ISO (3M Pharmaceuticals). | [Description]
Isoproterenol is a representative of the sympathomimetic drugs with high selectivity to β-
adrenoreceptors. As was already noted, the addition to compounds of a bulky iso-propyl or
tert-butyl group at the nitrogen atom of the β-phenylethylamino skeleton is associated with
higher affinity to β-adrenergic receptive regions than to α-adrenergic. Isoproterenol is
devoid of significant α-adrenergic agonistic action. Activation of β1-adrenergic receptors
in the heart increases positive chronotropic and ionotropic action. Peripheral vascular
resistance is increased by the widening of blood vessels, primarily in skeletal muscle, but
also in renal and mesenteric blood circulation, which is caused by the β2-adrenergic
system. | [Chemical Properties]
Colorless crystals with a melting point of 155.5°C. Its hydrochloride and sulfate salts are commonly used. Both are white crystalline powders, soluble in water but insoluble in chloroform or ether. Odorless with a slightly bitter taste. Its hydrochloride, [51-30-9], has a melting point of 170°C (decomposition); its sulfate, [6700-39-6], has a melting point of 128°C (decomposition). | [Originator]
Isonorin,Smith, Miller and Patch,US,1949 | [Indications]
Isoproterenol is administered almost exclusively by
inhalation from metered-dose inhalers or from nebulizers.
The response to inhaled isoproterenol and other inhaled
adrenomimetics is instantaneous. The action of
isoproterenol is short-lived, although an objective
measurement of pulmonary function has shown an effective
duration of up to 3 hours. When it is administered
by inhalation, the cardiac effects of isoproterenol
are relatively mild, although in some cases a substantial
increase in heart rate occurs.
| [Manufacturing Process]
As described in US Patent 2,308,232, 100 g 3,4-dihydroxy-ω-
chloroacetophenone, 200 cc ethyl alcohol and 200 cc of about 50% aqueous
isopropylamine solution are boiled during 3 hours on the water bath with the
use of a reflux condenser, whereupon neutralizing with diluted sulfuric acid is
carried out and the sulfate, obtained upon cooling, from alcohol of 50% is
recrystallized; its MP is 245°C.
21 g 3,4-dihydroxy-ω-isopropylaminoacetophenone sulfate are hydrogenated
with 50 cc methyl alcohol and 50 cc water, 0.5 g carbon and 3 cc palladium
chloride solution of 2%. After 2 hours the hydrogen absorption comes to a
standstill, after the theoretical quantity of hydrogen has been absorbed. After
concentrating, the isopropylaminomethyl-(3,4-dihydroxyphenyl)carbinolsulfate
crystallizes out. It has a MP of 180°C after refining. | [Therapeutic Function]
Bronchodilator | [World Health Organization (WHO)]
Isoprenaline, a beta-adrenoreceptor agonist, was introduced in
1949 as treatment for a number of cardiac disorders and as a bronchial dilator for
the symptomatic treatment of asthma. There is evidence that regular inhalation of
bronchodilator drugs is associated, in some cases with exacerbation of the
disease and with increased fatality rates. The underlying causes are disputed, but
an increasing body of opinion now advocates regular maintenance therapy with
inhaled, corticosteroids coupled with supplementary use as required of bronchial
drugs to suppress exacerbations. | [General Description]
Isoproterenol is a nonselective and prototypical -agonist ( β2/ β1=1). After oral administration,the absorption of ISO is rather erratic and undependable.The principal reason for its poor absorption characteristicsand relatively short DOA is its facile metabolism by sulfateand glucuronide conjugation of the phenolic OH groupsand O-methylation by COMT. Because it is a catechol, it issensitive to light and air. Aqueous solutions become pinkon standing. Unlike E and NE, ISO does not appear to undergo oxidative deamination by MAO. The drug hasDOA of 1 to 3 hours after inhalation. | [Clinical Use]
Isoproterenol is used principally by inhalation for
the management of bronchospasm. It is also used intravenously
for asthma and as a stimulant in cardiac arrest. | [Clinical Use]
The cardiac stimulation caused by its 1-activity andits lack of oral activity have led to its diminished use infavor of more selective -agonists. The problems have beenovercome at least partially by the design and developmentof several noncatechol selective 2-agonists. These agentsrelax smooth muscle of the bronchi, uterus, and skeletalmuscle vascular supply. They find their primary use as bronchodilatorsin the treatment of acute and chronic bronchialasthma and other obstructive pulmonary diseases. | [Synthesis]
Isoproterenol, 1-(3,4-dihydroxyphenyl)-2-iso-propylaminoethanol (11.1.8),
is synthesized by an analogous scheme of making epinephrine. Interaction of |?-chloro-3,4-
dihydroxyacetophenone (chloroacetylpyrocatechol) with isopropylamine gives |?-isopropy�lamino-3,4-dihydroxyacetophenone (11.1.7), reduction of the carbonyl group of which by
hydrogen using a palladium on carbon catalyst gives isoproterenol (11.1.8) [11,12].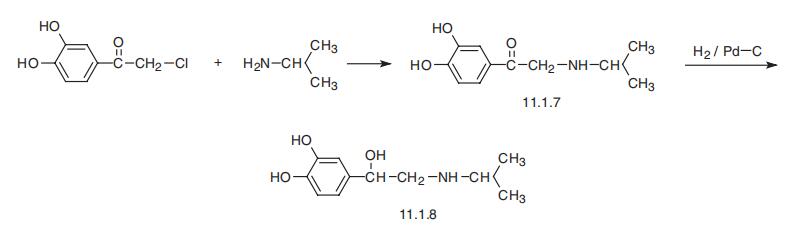
|
| Safety Data | Back Directory | [Safety Profile]
Poison by ingestion,
subcutaneous, intravenous, and
intraperitoneal routes. An experimental
teratogen. Other experimental reproductive
effects. Human systemic effects by
intramuscular route: increased pulse and
cardac rate. A bronchodilator. Mutation
data reported. When heated to
decomposition it emits toxic fumes of NOx |
|
|