| Identification | More | [Name]
2,3-DIAMINONAPHTHALENE | [CAS]
771-97-1 | [Synonyms]
2,3-DIAMINONAPHTHALENE
2,3-NAPHTHALENEDIAMINE
2,3-NAPHTHYLENEDIAMINE
DAN
TIMTEC-BB SBB000127
2,3-Diaminonaphthalene(ForNoDetection)
NAPHTHALENEDIAMINE
Naphthalene-2,3-diamine | [EINECS(EC#)]
212-241-0 | [Molecular Formula]
C10H10N2 | [MDL Number]
MFCD00004116 | [Molecular Weight]
158.2 | [MOL File]
771-97-1.mol |
| Chemical Properties | Back Directory | [Appearance]
colourless to white crystalline powder | [Melting point ]
197-203 °C
| [Boiling point ]
273.29°C (rough estimate) | [density ]
1.0968
| [refractive index ]
1.6392 (estimate) | [storage temp. ]
Refrigerator | [solubility ]
pyridine: soluble50mg/mL | [form ]
Crystalline Powder | [pka]
5.36±0.10(Predicted) | [color ]
Green-brown to brown | [Stability:]
Stable. Combustible. Incompatible with strong oxidizing agents. | [Water Solubility ]
Slightly soluble in water. Soluble in pyridine, dimethylsulfoxide, dimethyformamide, dil.hydrochloric acid, ethanol, ether and hot methanol. | [BRN ]
2206394 | [InChI]
InChI=1S/C10H10N2/c11-9-5-7-3-1-2-4-8(7)6-10(9)12/h1-6H,11-12H2 | [InChIKey]
XTBLDMQMUSHDEN-UHFFFAOYSA-N | [SMILES]
C1=C2C(C=CC=C2)=CC(N)=C1N | [Uses]
In a reaction similar to that of DAF-FM , 2,3- diaminonaphthalene (D-7918) reacts with the nitrosonium cation that forms spontaneously from NO to form the fluorescent product 1H-naphthotriazole.Using 2,3-diaminonaphthalene, researchers have developed a rapid, quantitative fluorometric assay that can detect from 10 nM to 10 μM nitrite and is compatible with a 96-well microplate format. | [CAS DataBase Reference]
771-97-1(CAS DataBase Reference) | [EPA Substance Registry System]
2,3-Naphthalenediamine (771-97-1) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [RIDADR ]
2811 | [WGK Germany ]
3
| [F ]
8-10-23 | [TSCA ]
Yes | [HS Code ]
29215900 | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Acute Tox. 4 Oral
Eye Irrit. 2
Skin Irrit. 2
STOT SE 3 |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Sodium hydroxide-->Dichloromethane-->N,N-Dimethylformamide-->Ammonium hydroxide-->Sodium hypochlorite-->1,1'-Carbonyldiimidazole-->3-Hydroxy-2-naphthoic acid-->Ammonium Sulfite-->2,3-Dihydroxynaphthalene-->1,6-Naphthalenediamine-->3-NITRO-2-NAPHTHYLAMINE-->N-{3-nitro-5,6,7,8-tetrahydro-2-naphthalenyl}acetamide-->3-AMINO-2-NAPHTHOL-->2,3-DICHLORONAPHTHALENE | [Preparation Products]
Atorvastatin-->6,13-dihydrodibenzo[b,i]phenazine-->1H-Naphth[2,3-d]imidazole,2-methyl-(7CI,9CI) |
| Hazard Information | Back Directory | [Description]
2,3-Diaminonaphthalene is a highly selective colorimetric and fluorometric reagent for selenium detection and also used for the fluorometric determination of nitrite. It is also useful for detection of ketones and alpha-keto acids.
2,3-Diaminonaphthalene reacts with nitrosonium, which is formed from nitrite at low pH, to form the fluorescent dye 1 H-naphthotriazole. This method can be used to detect 10 nM to 10 uM of nitrite (NO2-) and is compatible with 96-well format.
Fluorometric probe for nitrite
Quantify 10 nM to 10 uM of nitrite
Yellow orange solid soluble in DMSO | [Chemical Properties]
colourless to white crystalline powder | [Reactions]
2,3-diaminonaphthalene (DAN) is a fluorometric probe for nitrite. It reacts with nitrosonium, which is formed from nitrite at low pH, to form the fluorescent dye 1 H-naphthotriazole. This method can be used to detect 10 nM to 10 uM of nitrite (NO2-) and is compatible with 96-well format.
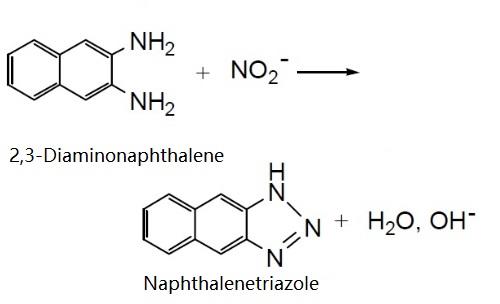
Reaction of 2,3-Diaminonaphthalene with NO2. | [Synthesis]
General procedure for the synthesis of 2,3-diaminonaphthalene from 2,3-dichloronaphthalene: Dissolve 2,3-dichloronaphthalene (2 g) in anhydrous ethanol. Ammonium chloride (1.5 g) was added and diluted. Subsequently, a catalytic amount of hydrochloric acid and an excess of concentrated aqueous ammonia solution were added to the reaction system and the reaction was refluxed on a water bath for 6 hours until the reaction solution showed a persistent dark green color. After completion of the reaction, the reaction solution was cooled and placed in a deep freezer overnight. Green crystals were obtained by filtration, dried and recrystallized with methanol. Dark green crystals were obtained; 75% yield; thin layer chromatography Rf values of 0.89 and 0.82; melting point 150 °C; IR spectra (νmax, KBr, cm-1): 758, 854, 1473 (aromatic C-N stretching vibration), 1500 (aromatic C=C stretching vibration), 1681 (N-H bending vibration), 3049 (N-H stretching vibration); 1H NMR (CDCl3, 500 MHz): δ 7.45-7.46 (dd, J=3.5-4 Hz, 2H, Ar-H), 7.12-7.14 (dd, J=3-3.5 Hz, 2H, Ar-H), 7.09 (s, 2H, Ar-H), 7.05 (s, 4H, Ar-NH2). | [Purification Methods]
Crystallise the diamine from water, or dissolve it in 0.1M HCl, by heating to 50o. After cooling, the solution is extracted with decalin to remove fluorescent impurities and centrifuged. [Beilstein 13 IV 346.] | [References]
[1] Oriental Journal of Chemistry, 2017, vol. 33, # 2, p. 821 - 828 |
|
|