| Identification | More | [Name]
2,4-Dihydroxy-3-nitropyridine | [CAS]
89282-12-2 | [Synonyms]
2,4-DIHYDROXY-3-NITROPYRIDINE
3-NITRO-2,4-PYRIDINEDIOL
4,6-DIHYDROXY-5-NITRO PYRIDINE
4-Hydroxy-3-nitro-2-pyridone
2,4-Hydroxy-3-nitropyridine.
2(1H)-Pyridinone, 4-hydroxy-3-nitro-
2,4-Dihydroxy-3-nitropyridine ,99% | [Molecular Formula]
C5H4N2O4 | [MDL Number]
MFCD01075671 | [Molecular Weight]
156.1 | [MOL File]
89282-12-2.mol |
| Chemical Properties | Back Directory | [Melting point ]
265°C (dec.) | [Boiling point ]
305.6±42.0 °C(Predicted) | [density ]
1.65±0.1 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,Room Temperature | [form ]
powder to crystal | [pka]
4.50±1.00(Predicted) | [color ]
Light yellow to Yellow to Green | [Detection Methods]
HPLC | [BRN ]
149558 | [InChI]
InChI=1S/C5H4N2O4/c8-3-1-2-6-5(9)4(3)7(10)11/h1-2H,(H2,6,8,9) | [InChIKey]
BKYGVGWYPFVKTK-UHFFFAOYSA-N | [SMILES]
C1(=O)NC=CC(O)=C1[N+]([O-])=O | [CAS DataBase Reference]
89282-12-2(CAS DataBase Reference) |
| Questions And Answer | Back Directory | [Uses]
2,4-Dihydroxy-3-nitropyridine can be used as a material additive, pharmaceutical chemical, and organic synthesis intermediate. During synthetic transformation, the hydroxyl groups in the structure can be easily converted into halogen atoms due to the influence of the pyridine ring and nitro group. It should be noted that by changing the reaction conditions, one of the two hydroxyl groups can be selectively halogenated, or both can be converted simultaneously. Furthermore, under acidic conditions, a bromine atom can be introduced at the 5-position of the pyridine using liquid bromine. |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [RIDADR ]
3335 | [HS Code ]
29333990 |
| Hazard Information | Back Directory | [Chemical Properties]
Yellow powder | [Reactions]
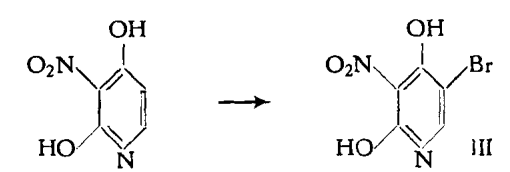
2,4-Dihydroxy-3-nitropyridine could be quickly transformed into 5-bromo-2,4-dihydroxy-3-nitropyridine by the action of a solution of bromine. 3.5 g of 2,4-Dihydroxy-3-nitropyridine were heated with a mixture of 1.15 ml of bromine and 30 ml of acetic acid at 70℃ for 15 minutes. When the reaction mixture was cooled with ice, 3.7 g of 5-bromo-2,4-dihydroxy-3-nitropyridine separated out[1]. Yield: 70 %
 | [Synthesis]
Step 59a. Synthesis of 2,4-dihydroxy-3-nitropyridine (compound 0108): fuming nitric acid (90 mL) was slowly added to a stirring solution of 2,4-dihydroxypyridine (0601) (100 g, 0.9 mol) dissolved in ethyl acetate (300 mL) at 0 °C. After 45 minutes of reaction, the reaction solution was carefully poured into crushed ice and the mixture was cooled in a refrigerator. The resulting precipitate was collected by filtration, washed with cold water and dried to give 4-hydroxy-3-nitropyridin-2(1H)-one (0108) (135 g, 96% yield) as a light yellow solid.LCMS: 157 [M + 1]+; 1H NMR (DMSO-d6) δ 6.05 (d, 1H, J = 7.2 Hz), 7.47 (d, 1H, J = 7.2 Hz), 11.91 (s, 1H), 12.47 (s, 1H). | [References]
[1] Kolder, C. R. , and H. J. D. Hertog . Migration of halogen atoms in halogeno-derivatives of 2,4-dihydroxypyridine (II). Recueil des Travaux Chimiques des Pays-Bas 72.10(2010):853-858. |
|
|