| Identification | More | [Name]
6-Dimethylaminopurine | [CAS]
938-55-6 | [Synonyms]
4DAMPY
4-DIMETHYLAMINOPYRIDINE
4-(N,N-DIMETHYLAMINO)-PYRIDINE
6-DIMETHYLAMINOPURINE
6 DMAP
DIMETHYLAMINO PYRIDINE
DIMETHYLAMINOPYRIDINE, 4-
DMAP
N4,N4-DIMETHYLPYRIDIN-4-AMINE
N-(4-PYRIDYL)DIMETHYLAMINE
N6,N6-DIMETHYLADENINE
N,N'-DIMETHYL-4-PYRIDINAMINE
N,N-DIMETHYL-N-(4-PYRIDINYL)AMINE
N,N-Dimethylpyridin-4-amine
TIMTEC-BB SBB008765
6-Dimethyladenine
6-Dimethylamino-9H-purine
Adenine, N,N-dimethyl-
Adenine, N6,N6-dimethyl-
Dimethyladenine | [EINECS(EC#)]
214-353-5 | [Molecular Formula]
C7H9N5 | [MDL Number]
MFCD00005573 | [Molecular Weight]
163.18 | [MOL File]
938-55-6.mol |
| Chemical Properties | Back Directory | [Appearance]
white to light yellow crystal powder | [Melting point ]
259-262 °C(lit.)
| [Boiling point ]
162 °C (50 mmHg)
| [density ]
1.1407 (rough estimate) | [refractive index ]
1.6380 (estimate) | [Fp ]
110 °C
| [storage temp. ]
−20°C
| [solubility ]
methanol: 0.1 g/mL, clear
| [form ]
prilled
| [pka]
9.38±0.20(Predicted) | [color ]
off-white to yellow
| [biological source]
synthetic (organic) | [Water Solubility ]
water: 50mg/mL, clear to hazy, colorless to light yellow | [Usage]
A purine antagonist. In the benzodiazepine receptor (BZR) binding assay, it inhibits the binding of 1.5 nM [3H]diazepam at 100uM in rat brains | [BRN ]
7634 | [InChIKey]
BVIAOQMSVZHOJM-UHFFFAOYSA-N | [CAS DataBase Reference]
938-55-6(CAS DataBase Reference) | [NIST Chemistry Reference]
1H-Purin-6-amine, N,N-dimethyl-(938-55-6) |
| Safety Data | Back Directory | [Hazard Codes ]
T+ | [Risk Statements ]
R25:Toxic if swallowed.
R27:Very Toxic in contact with skin.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S28:After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S24/25:Avoid contact with skin and eyes .
S22:Do not breathe dust . | [RIDADR ]
UN 2811 6.1/PG 1
| [WGK Germany ]
3
| [RTECS ]
US9230000
| [F ]
10-23 | [HS Code ]
29335990 |
| Hazard Information | Back Directory | [Chemical Properties]
white to light yellow crystal powder | [Uses]
A purine antagonist. In the benzodiazepine receptor (BZR) binding assay, it inhibits the binding of 1.5 nM [3H]diazepam at 100uM in rat brains | [Application]
6-(Dimethylamino)purine has been used:
as a supplement in GR-1 aa medium (bovine medium) for parthenogenetic activation of bovine oocytes to study its potential for embryo development.
in the activation step during the production of nuclear transfer embryos.
as a supplement in HCR2aa medium to activate interspecies embryos derived from interspecies somatic cell nuclear transfer (iSCNT) technique.
A purine antagonist.
In the benzodiazepine receptor (BZR) binding assay, it inhibits the binding of 1.5 nM [3H]diazepam at 100uM in rat brains. | [Definition]
ChEBI: Adenine substituted at N-6 by geminal methyl groups. | [Preparation]
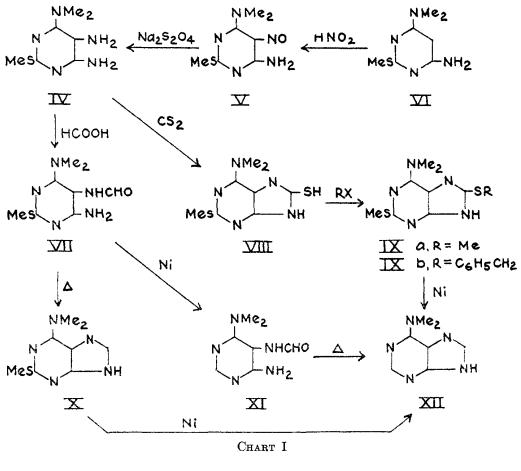
6-Dimethylaminopurine synthesis: 2-Methylmercapto-4-amino-6-dimethylaminopyrimidine (VI) was smoothly nitrosated in 10% acetic acid to the 5-nitrosopyrimidine (V) in 95% yield. Reduction of V with sodium hydrosulfite to the triamine (IV), followed by formylation gave the 5-formamidopyrimidine (VII) in 76% over-all yield for the two steps. Reductive formylation of V directly to VI1 with zinc and formic acid, although more rapid, was less efficient (50% yield). Ring closure of VII to 2-methyhercapto-6-dimethylaminopurine (X) was best done on a small scale by short fusion at 250°(99% yield), although boiling quinoline, formamide, or dilute alcoholic sodium hydroxide could also be employed. The latter reagent was most efficient on a large scale. Desulfurization of X with Raney nickel (7) in 1 N sodium hydroxide at 100° afforded the final product, 6-dimethylaminopurine (XII) in 43% yield.This compound was identical in all respects with the C7H9N5 moiety from puromycin (2).
 | [Origin]
6-Dimethylaminopurine is a puromycin analog that was first identified in the spores of Streptomyces alboniger (PMID: 5019066 ). It has subsequently been identified in several algae species (PMID: 4206669 ).
| [General Description]
6-(Dimethylamino)purine (6-DMAP) is a purine-based metabolite with two condensed heterocyclic rings and two methyl groups linked to the amino group of the purine unit of adenine. | [Biochem/physiol Actions]
6-(Dimethylamino)purine (6-DMAP) is a protein kinase and cyclin-dependent kinase inhibitor. It acts as a secondary metabolite and mediates RNA modification. 6-DMAP is a potent cytokinetic inhibitor and is used in parthenogenesis and meiosis studies. It is also used to promote pronuclei formation in mammalian oocytes. 6-DMAP is a dual fluorescence molecule according to femtosecond fluorescence up-conversion spectroscopy studies. | [Synthesis]
The general procedure for the synthesis of N,N-dimethyl-7H-purin-6-amine from N,N-dimethylformamide and 6-chloropurine is as follows:
1. a reaction solution was prepared by dissolving 6-chloropurine (1.10 g, 4.5 mmol), N,N-dimethylformamide (244 mg, 1 mmol), catalyst 3 (649 mg, 2.5 mmol), additive 4 (518 mg, 2 mmol) and additive 5 (310 mg, 2 mmol) in DMF (6 mL).
2. the reaction solution was dispensed into multiple vials, each containing the starting compound (0.5 mmol) and DMF (6 mL).
3. Heat the solution in each vial in a microwave reactor.
4. Upon completion of the reaction, the reaction mixtures were combined and the solvent was evaporated under vacuum.
5. for products 10, 11, 12, 13 and 16, the residue was purified by rapid chromatographic separation on a silica gel column.
6. for products 14 and 15, the residue was dispersed in a solvent mixture of water (60 mL) and ethyl acetate (EtOAc, 50 mL).
7. The aqueous layer was extracted with ethyl acetate (3 x 40 mL), the organic phases were combined and dried with anhydrous magnesium sulfate (MgSO4), filtered and the solvent was evaporated to give the target product. | [storage]
Store at -20°C | [Purification Methods]
It is purified by recrystallisation from H2O, EtOH (0.32g in 10mL) or CHCl3. [Albert & Brown J Chem Soc 2060 1954, UV: Mason J Chem Soc 2071 1954.] The monohydrochloride crystallises from EtOH/Et2O, m 2 5 3o(dec) [Elion et al. J Am Chem Soc 74 411 1952], the dihydrochloride has m 225o(dec) and the picrate has m 245o (235-236.5o) [Fryth et al. J Am Chem Soc 80 2736 1958]. [Beilstein 26 III/IV 3566.] | [References]
[1] Synthetic Communications, 2004, vol. 34, # 16, p. 2925 - 2930
[2] Tetrahedron, 2011, vol. 67, # 5, p. 866 - 871 |
|
|