123171-59-5
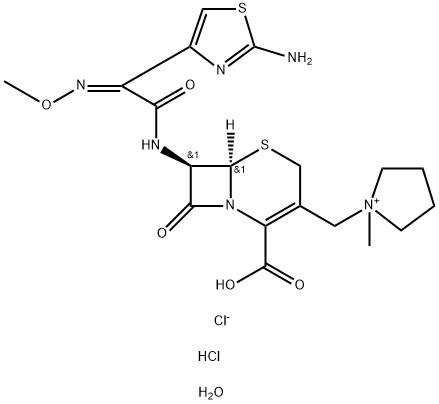 123171-59-5 结构式
123171-59-5 结构式
基本信息
氨噻肟酸苯并三唑酯(活性氧酯)
(6R,7R)-7-[(Z)-2-(2-氨基-4-噻唑基)-2-甲氧亚胺]乙酰胺基-3-[1-(1-甲基吡咯烷基)甲基]-8-氧-5-硫杂-1-氮杂双环[4.2.0]辛-2-烯-2-羧酸盐酸盐一水合物
头孢吡肟盐酸盐
盐酸头孢吡肟
Pyrrolidinium, 1-[[(6R,7R)-7-[[(2Z)-(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-1-methyl-, chloride, monohydrochloride, monohydrate
Pyrrolidinium, 1-[[7-[[(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-1-methyl-, chloride, monohydrochloride, monohydrate, [6R-[6α,7β(Z)]]-
(6R,7R)-7-[(Z)-2-(2-Amino-4-thiazolyl)-2-(methoxyimino)acetamido]-3-[1-(1-methylpyrrolidinyl)methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-2-carboxylic acid hydrochloride monohydrate
Cefepime hydrochloride
CEFEPIME HYDROCHLORIDE (BUFFERED WITH ARGININE)
1-[[(6R,7R)-7-[[(2Z)-(2-Amino-4-thiazolyl)(methoxyimino)acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-1-methyl- pyrrolidinium Inner Salt Hydrochloride Monohydrate
Axepim
Cefepime Hydrochloride Monohydrate
Cepimex
Maxipime
物理化学性质
安全数据
常见问题列表
盐酸头孢吡肟即头孢吡肟二氯化氢盐一水合物。头孢吡肟(cefepime)是第四代头孢类注射用抗生素,对革兰氏阴性菌、革兰氏阳性菌和厌氧菌均具有良好的抗菌活性,同时对β-内酰胺酶表现出较高的稳定性,较第三代头孢抗生素具有更为广泛的抗菌谱和抗菌活性。
临床上用于治疗严重感染。盐酸头孢吡肟是在弱酸性条件下沉淀钠盐,有效避免了头孢吡肟碱性条件下分解和颜色加深的问题,提高了产品质量。
常温下(15~25℃)把171g异辛酸钠(1.03mol)溶于900mL水和200mL丙酮中,有效搅拌下分批加入300g硫酸头孢吡肟(0.52mol,重量按无水物计),形成流动性良好的浆状体系。10分钟后加入1600mL丙酮和适量活性炭,搅拌30分钟。离心或抽滤分离出固体。用丙酮-水2∶1(v/v)混合溶液500mL洗涤滤饼,合并滤液。搅拌下往滤液中加入18%HCl至pH1.0~1.5。然后1小时内加入8L丙酮。体系再降温至5~10℃,继续搅拌1小时。抽滤。滤饼用丙酮洗涤,40℃下真空干燥,得约270g白色结晶性粉末。纯度99.7%,含水量为3.8%(K-F法),丙酮残留0.25%。产品是头孢吡肟二氯化氢盐一水合物,即盐酸头孢吡肟。所有质量指标符合美国药典要求。
头孢吡肟盐酸盐为盐酸头孢吡肟加适量L-精氨酸制成的无菌混合物,为白色至淡黄色粉末,几乎无臭,有引湿性。体外试验表明,本品对革兰氏阳性菌和阴性菌均有作用。本品对细菌染色体编码的β-内酰胺酶的亲和力低,可高度耐受多数β-内酰胺酶的水解,并可迅速渗入革兰阴性菌的细胞内。在菌体细胞内,其靶分子为青霉素结合蛋白(PBP)。
盐酸头孢吡肟是广谱的头孢菌素,增强了对革兰氏阳性菌和革兰氏阴性菌的范围。
Antibacterial
Cefepime is an extended-spectrum parenteral cephalosporin antibiotic active in vitro against a broad spectrum of gram-positive and gram-negative aerobic bacteria. Cefepime has a decreased propensity to induce beta-lactamases compared with other beta-lactam antibiotics.
Cefepime has a pharmacokinetic disposition similar to that of other renally eliminated cephalosporins, with a half-life of approximately 2 hours. Cefepime has demonstrated clinical efficacy against a variety of infections, including urinary tract infections, pneumonia, and skin and skin structure infections. Cefepime is generally well tolerated.
