132684-60-7
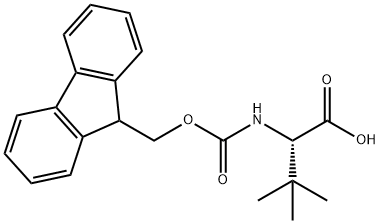 132684-60-7 结构式
132684-60-7 结构式
基本信息
N-芴甲氧羰基-L-叔亮氨酸
FMOC-ALPHA-T-BUTYL-GLY-OH
FMOC-L-ALPHA-T-BUTYLGLYCINE
FMOC-L-T-BUTYLGLYCINE
FMOC-L-TERT-LEUCINE
FMOC-L-TLE-OH
FMOC-TBU-GLYCINE
FMOC-TBU-GLY-OH
FMOC-TLE-OH
N-ALPHA-(9-FLUORENYLMETHOXYCARBONYL)-L-ALPHA-T-BUTYLGLYCINE
N-ALPHA-(9-FLUORENYLMETHOXYCARBONYL)-L-TERT-LEUCINE
N-ALPHA-(9-FLUORENYLMETHOXYCARBONYL)-L-T-LEUCINE
N-ALPHA-(9-FLUORENYLMETHOXYCARBONYL)-(S)-2-AMINO-3,3-DIMETHYL-BUTYRIC ACID
N-ALPHA-(9-FLUORENYLMETHYLOXYCARBONYL)-L-TERT-BUTYL-GLYCINE
N-ALPHA-(9-FLUORENYLMETHYLOXYCARBONYL)-L-T-LEUCINE
N-ALPHA-FMOC-L-ALPHA-T-BUTYL-GLYCINE
N-ALPHA-FMOC-L-T-BUTYLGLYCINE
N-ALPHA-FMOC-L-T-LEUCINE
N-ALPHA-T-FMOC-L-ALPHA-T-BUTYL-GLYCINE
N-ALPHA-T-FMOC-L-T-LEUCINE
物理化学性质
制备方法
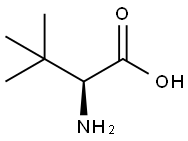
20859-02-3
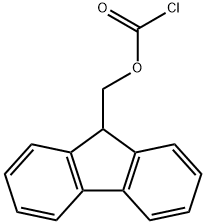
28920-43-6

132684-60-7
以L-叔亮氨酸和氯甲酸-9-芴基甲酯为原料合成Fmoc-L-叔亮氨酸的一般步骤:取一个500mL的反应瓶,向其中加入L-叔亮氨酸(5.1g,38.6mmol)、二恶烷(40mL)和10%碳酸钠溶液(100mL)。将反应瓶置于冰浴中,在机械搅拌下,通过滴液漏斗缓慢加入氯甲酸-9-芴基甲酯(10.0g,38.6mmol)的二恶烷溶液(100mL)。滴加完毕后,逐渐恢复至室温并继续搅拌过夜。反应完成后,加入100mL水,用50mL乙醚萃取三次,保留水相。将水相置于冰浴中冷却,缓慢加入1M稀盐酸调节pH至1。随后,用50mL乙酸乙酯萃取水溶液三次。合并有机相,用无水硫酸镁干燥,过滤后浓缩至干,得到中间体Fmoc-L-叔亮氨酸(14.3g,收率96%)。
参考文献:
[1] Patent: CN106588987, 2017, A. Location in patent: Paragraph 0195; 0196; 0197
[2] Angewandte Chemie - International Edition, 2013, vol. 52, # 45, p. 11846 - 11851
[3] Angew. Chem., 2013, vol. 125, # 45, p. 12062 - 12067,6
[4] Chemistry - A European Journal, 2014, vol. 20, # 21, p. 6526 - 6531
[5] Patent: WO2018/145021, 2018, A1. Location in patent: Page/Page column 134
