16652-71-4
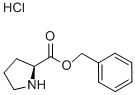 16652-71-4 结构式
16652-71-4 结构式
基本信息
H-PRO-OBZL·HCL
脯氨酸苯酯盐酸盐
脯氨酸苄酯盐酸盐
H-PRO-OBZL HCL
L-PROLINE BENZYL ESTER
L-PROLINE BENZYL ESTER HCL
L-PROLINE BENZYL ESTER HYDROCHLORIDE SALT
L-PROLINE BENZYL ESTER MONOHYDROCHLORIDE
L-PYRROLIDINE-2-CARBOXYLIC ACID BENZYL ESTER HCL
PROLINE-OBZL HCL
PRO-OBZL HCL
TIMTEC-BB SBB003206
l-proline,phenylmethylester,hydrochloride
Proline benzyl ester hydrochloride
benzyl L-prolinate hydrochloride
L-PROLINE BENZYL ESTER HYDROCHLORIDE, 98 %
H-L-Pro-OBzl*HCl
L-Pro-OBzl.HCl
物理化学性质
安全数据
制备方法
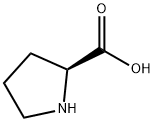
147-85-3

100-51-6

16652-71-4
在氮气保护下,将苄醇(70 mL,651 mmol)冷却至0℃,缓慢滴加亚硫酰氯(7.0 mL,91.2 mmol)。随后加入L-脯氨酸(5.0 g,43.4 mmol),保持反应体系在0℃及氮气氛围下搅拌2小时。缓慢升温至室温,继续搅拌48小时。反应完成后,将反应混合物缓慢倒入乙醚(300 mL)中,并于-20℃静置结晶7天。过滤收集所得白色固体沉淀,用乙醚洗涤,真空干燥,得到L-脯氨酸苄酯盐酸盐(9.88 g,收率93%)。产物表征数据如下:熔点142.1-144.0℃(文献值:143-144℃);1H NMR (300 MHz, CDCl3): δ 7.41-7.21 (m, 5H), 5.16 (s, 2H), 3.80 (dd, J = 3.83, 5.9 Hz, 1H), 3.15-3.01 (m, 1H), 3.00-2.82 (m, 1H), 2.42-2.21 (m, 1H), 2.13 (dd, J = 12.9, 7.5 Hz, 1H), 1.92-1.62 (m, 3H);13C NMR (75 MHz, CDCl3): δ 175.5, 136.0, 128.8, 128.5, 128.3, 66.9, 59.9, 47.2, 30.4, 25.6。元素分析(C12H16ClNO2)计算值:C, 59.63; H, 6.67; N, 5.79;实测值:C, 59.50; H, 6.86; N, 5.64。
参考文献:
[1] Bioorganic and Medicinal Chemistry, 2015, vol. 23, # 15, p. 5056 - 5060
[2] Tetrahedron, 2010, vol. 66, # 29, p. 5384 - 5395
[3] Patent: CN105061283, 2017, B. Location in patent: Paragraph 0030-0032; 0045-0047; 0057
[4] Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, 1959, p. 1851; engl. Ausg. S. 1768, 1769
[5] Journal of Biological Chemistry, 1951, vol. 193, p. 97,108
