446292-07-5
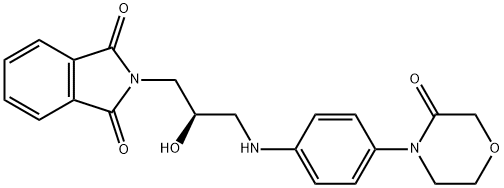 446292-07-5 结构式
446292-07-5 结构式
基本信息
利伐沙班杂质39
利伐沙班杂质50
利伐沙班脱羰基杂质
利伐沙班氧代邻苯二甲酰亚胺脱羰基杂质
4-[((2R)-羟基-3-邻苯二甲酰亚胺)丙胺]苯基-3-吗啉
氢-异吲哚-1,3(2H)-二酮,2-[(2R)的-2-羟基-3-[[4-(3-氧-4-吗啉基
2-[(2R)-2-羟基-3-[[4-(3-氧代-4-吗啉基)苯基]氨基]丙基]-1H-异吲哚-1,3(2H)-二酮
2-[(2R)-2-羟基-3-[[4-(3-氧代-4-吗啉基)苯基]氨基]丙基]-1H-异吲哚-1,3(2H)-二酮,利伐沙班中间体
2-[(2R)-2-羟基-3-[[4-(3-氧代-4-吗啉基)苯基]氨基]丙基]-1H-异吲哚-1,3(2H)-二酮,利伐沙班中间体 1KG
Rivaroxaban interMediate B
-2-(2-Hydroxy-3-((4-(3-oxomorpholino)
Rivaroxaban Phthalimido Descarbonyl Impurity
4-[((2R)-Hydroxy-3-phthaliMido)propylaMine]phenyl-3-Morpholinone
2-[(2R)-2-HYDROXY-3-[[4-(3-OXO-4-MORPHOLINYL)PHENYL]AMINO]PROPYL]-
2-[(2R)-2-hydroxy-3-[4-(3-oxomorpholin-4-yl)anilino]propyl]isoindole-1,3-dione
(R)-2-(2-Hydroxy-3-((4-(3-oxomorpholino)phenyl)-amino)propyl)isoindoline-1,3-dione
2-[(2R)-2-Hydroxy-3-[[4-(3-oxo-4-morpholinyl)phenyl]amino]propyl]-1H-isoindole-1,3(2H)-dione
2-[(2R)-2-hydroxy-3-{[4-(3-oxoMorpholin-4-yl)phenyl]aMino}p ropyl]-1H-isoindole-1,3(2H)-dione
物理化学性质
制备方法
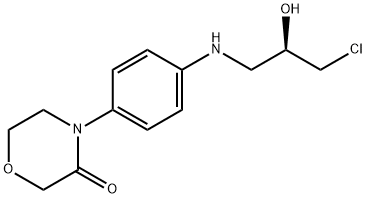
1252018-10-2
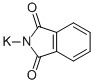
1074-82-4

446292-07-5
实施例3:(R)-2-(2-羟基-3-((4-(3-氧代吗啉)苯基)氨基)丙基)异吲哚啉-1,3-二酮(7a)的制备:将邻苯二甲酰亚胺钾盐(7.16 g, 38.632 mmol)一次性加入到机械搅拌的4-[4-(N-(3-氯-2R-羟基-1-丙基)氨基)苯基]吗啉-3-酮(10 g, 35.119 mmol)的N,N-二甲基甲酰胺(DMF, 60 mL)溶液中。将悬浮液在室温下搅拌后,加热至100℃,并在该温度下持续搅拌3小时。反应完成后,将混合物冷却至室温。加入水(60 mL)并将悬浮液继续搅拌15分钟。随后,将悬浮液通过布氏漏斗过滤,收集固体产物。用蒸馏水(2×40 mL)洗涤固体,并在50℃下真空干燥10小时,得到目标产物(R)-2-(2-羟基-3-((4-(3-氧代吗啉)苯基)氨基)丙基)异吲哚啉-1,3-二酮(12.55 g, 产率93%),为白色结晶固体。1H NMR (400 MHz, DMSO-d6) δ: 2.99-3.05 (m, 1H), 3.14-3.2 (m, 1H), 3.59-3.69 (m, 4H), 3.91-3.94 (m, 2H), 3.97-4.05 (m, 1H), 4.14 (s, 2H), 5.16 (d, J = 5.2 Hz, 1H), 5.66 (t, J = 6.0 Hz, 1H), 6.61 (d, J = 8.7 Hz, 2H), 7.03 (d, J = 8.8 Hz, 2H), 7.82-7.88 (m, 4H)。
参考文献:
[1] Patent: WO2012/51692, 2012, A1. Location in patent: Page/Page column 42-43
[2] Patent: CN103951661, 2017, B. Location in patent: Paragraph 0043-0044
[3] Patent: CN104974105, 2017, B. Location in patent: Paragraph 0073
[4] Patent: CN104974149, 2018, B. Location in patent: Paragraph 0074; 0075; 0077; 0081; 0083
[5] Patent: WO2012/32533, 2012, A2. Location in patent: Page/Page column 15
