898543-06-1
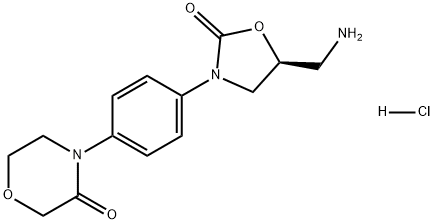 898543-06-1 结构式
898543-06-1 结构式
基本信息
利伐沙班中间体5
利伐沙班杂质27
利伐沙班杂质 7-2
利伐沙班 中间体 R-7.2
4-[4-[(5S)-5-(氨基甲基)-2-氧代-3-恶唑烷...
(S)-4-(4-(5-(氨基甲基)-2-氧代恶唑烷-3-基)苯基)吗啉-3-酮盐酸盐
4-[4-[(5S)-5-(氨甲基)-2-氧代-3-恶唑烷基]苯基]-3-吗啉酮盐酸盐
4-[4-[(5S)-5-(氨甲基)-2-羰基-3-唑烷基]苯基]-3-吗啡啉酮盐酸盐
(S)-4-(4-(5-(氨基甲基)-2-氧代噁唑烷-3-基)苯基)吗啉-3-酮盐酸盐
Rivaroxaban Impurity 7-2
Rivaroxaban interMediate A
4-{4-[(5S)-5-aMinoMethyl)-2-oxo-1,3-oxazolidin-3-yl}Morpholin-3-one
(S)-4-(4-(5-(Aminomethyl)-2-oxoxazolidin-3-yl)phenyl)morpholin-3-one.HCl
(S)-4-(4-(5-(Aminomethyl)-2-oxooxazolidin-3-yl)phenyl)morpholin-3-one.HCl
4-(4-(5-(Aminomethyl)-2-oxooxazolidin-3-yl)phenyl)morpholin-3-one hydrochloride
(S)-4-(4-(5-(aMinoMethyl)-2-oxooxazolidin-3-yl)phenyl)Morpholin-3-one hydrochloride
4-{4-[(5S)-5-aMinoMethyl)-2-oxo-1,3-oxazolidin-3-yl} Mor-pholin -3-one hydrochloride
4-[4-[(5S)-5-(Aminomethyl)-2-oxo-3-oxazolidinyl]phenyl]-3-morpholinone hydrochloride
物理化学性质
制备方法
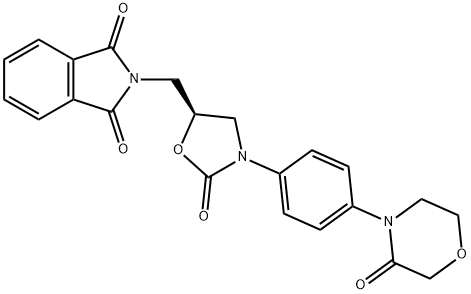
446292-08-6

898543-06-1
以2-[[(5S)-2-氧代-3-[4-(3-氧代-4-吗啉基)苯基]-5-噁唑烷基]甲基]-1H-异吲哚-1,3(2H)-二酮为原料,合成(S)-4-(4-(5-(氨基甲基)-2-氧代噁唑烷-3-基)苯基)吗啉-3-酮盐酸盐的一般步骤如下:在四颈圆底烧瓶中,加入甲醇(2900 mL),2-[[(5S)-2-氧代-3-[4-(3-氧代-4-吗啉基)苯基]-5-噁唑烷基]甲基]-1H-异吲哚-1,3(2H)-二酮(290 g)和40%甲胺水溶液(265 g)。将反应混合物在25至30℃下搅拌1小时,随后加热至60至65℃,并在该温度下维持4小时。反应完成后,将混合物冷却至25至30℃,加入浓盐酸(290 mL,调节pH至1-2),继续搅拌30分钟。过滤收集所得固体,并用冷甲醇(290 mL)洗涤。产率为92.0%。
参考文献:
[1] Patent: WO2014/102820, 2014, A2. Location in patent: Paragraph 059
[2] Patent: CN105085371, 2017, B. Location in patent: Paragraph 0102-0104
[3] Patent: CN105085431, 2017, B. Location in patent: Paragraph 0093; 0094; 0095; 0096; 0097; 0098; 0099
[4] Patent: WO2012/35057, 2012, A2. Location in patent: Page/Page column 29
[5] Patent: WO2013/121436, 2013, A2. Location in patent: Page/Page column 27
