611-53-0
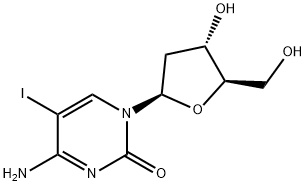 611-53-0 结构式
611-53-0 结构式
基本信息
伊巴他滨
5-碘-2'-脱氧胞苷
5-IDC
5-IODO-2'-DEOXYCYTIDINE
IBACITABINE
IDC
2'-Deoxy-5-iodo-D-cytidine
5-IODO-2''-DEOXYCYTIDINE(5-IDC)
5-IODO-2''-DEOXYCYTIDINE HPLC 99%
4-Amino-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-iodopyrimidin-2-one
2''-DEOXY-5-IODOCYTIDINE (5-IODO-2''-DEOXYCYTIDINE: 5-IDC)
Idoxcitidin
NSC-527083
物理化学性质
制备方法
951-77-9

611-53-0
以4-氨基-1-((2R,4S,5R)-4-羟基-5-(羟甲基)四氢呋喃-2-基)嘧啶-2(1H)-酮(dC)为原料合成4-氨基-1-((2R,4S,5R)-4-羟基-5-(羟甲基)四氢呋喃-2-基)-5-碘嘧啶-2(1H)-酮的一般步骤:在火焰干燥的圆底烧瓶中,将10.0 g dC(44.0 mmol,1.0当量)、7.70 g碘(26.4 mmol,0.6当量)和11.4 g mCPBA(70%,46.2 mmol,1.05当量)溶解于120 mL DMF中。反应混合物在室温下搅拌2小时,随后蒸发至干(在随后的柱色谱法中可接受少量DMF残留)。通过柱色谱法纯化(洗脱剂比例:DCM/MeOH/H2O/NH3 190:10:0.6:0.6 → 90:10:0.6:0.6),得到9.71 g(产率63%)目标化合物1,为橙色固体。产物表征数据如下:1H NMR (400 MHz, CDCl3/MeOD) δ (ppm) = 8.46 (s, 1H), 6.13 (t, 3J = 6.0 Hz, 1H), 4.34 (dt, 3J = 4.7, 6.3 Hz, 1H), 3.93 (dt, 3J = 3.0, 4.3 Hz, 1H), 3.84 (dd, 3J = 3.0, 2J = 12.1 Hz, 1H), 3.72 (dd, 3J = 3.2, 2J = 12.1 Hz, 1H), 2.39 (ddd, 3J = 4.8, 6.3, 2J = 13.7 Hz, 1H), 2.20-2.09 (m, 1H)。13C NMR (101 MHz, MeOD) δ (ppm) = 163.9, 153.9, 150.9, 89.5, 88.3, 71.5, 62.2, 56.2, 42.5。HRMS (ESI+) 计算值 C9H13IN3O4+ [M+H]+: 353.9945,实测值: 353.9944。熔点范围: 133-135°C(分解)。IR (ATR): 3191 (w), 1718 (m), 1642 (s), 1286 (m), 1087 (s), 957 (s), 750 (m)。
参考文献:
[1] Organic Letters, 2010, vol. 12, # 24, p. 5671 - 5673
[2] Patent: WO2012/62907, 2012, A1. Location in patent: Page/Page column 13; 14
[3] Patent: US2013/237697, 2013, A1. Location in patent: Paragraph 0052-0055
[4] Synthesis, 2009, # 23, p. 3957 - 3962
[5] Patent: US2012/178919, 2012, A1. Location in patent: Page/Page column 4
