96568-07-9
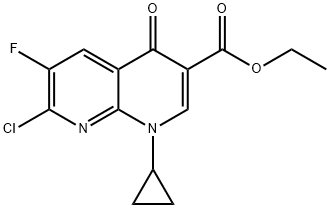 96568-07-9 结构式
96568-07-9 结构式
基本信息
环丙基萘啶羧酸乙酯 100G
环丙基萘啶羧酸乙酯:吉米沙星母核-2
1-环丙基-6-氟-7-氯-4-氧-1,4-二氢-1,8-萘啶-3-羧酸乙酯
1-环丙基-4-氧代-6-氟-7-氯-1,4-二氢-1,8-萘啶-3-甲酸乙酯
Ethyl 1-Cyclopropyl-
7-Chloro-1-cyclopropyl-6-fluoro-1
8-naphthyridine-3-carboxylic Acid Ethyl Ester
Ethyl 1-Cyclopropyl-6-Fluoro-7-Chloro-4-Oxo-1,
ethyl 7-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,8-naphthyridine-3-carboxylate
Ethyl1-Cyclopropyl-7-chloro-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthylrideincarboxylate
ETHYL 1-CYCLOPROPYL-7-CHLORO-6-FLUORO-1,4-DIHYDRO-4-OXO-1,8-NAPHTHYLRIDINE CARBOXYLATE
Ethyl 1-cyclopropyl-6-fluoro-7-chloro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylate
Ethyl 7-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylate
物理化学性质
制备方法
![3-Pyridinepropanoic acid, 2,6-dichloro-α-[(cyclopropylamino)methylene]-5-fluoro-β-oxo-, ethyl ester](/CAS/20211123/GIF/96568-06-8.gif)
96568-06-8

96568-07-9
以3-环丙基氨基-2-(2,6-二氯-5-氟吡啶-3-羰基)丙烯酸乙酯(CAS:96568-06-8)为原料合成7-氯-1-环丙基-6-氟-1,4-二氢-4-氧代-1,8-二氮杂萘-3-羧酸乙酯的一般步骤如下:在75-80℃下,将7.0 g 3-环丙基氨基-2-(2,6-二氯-5-氟吡啶-3-羰基)丙烯酸乙酯溶于35 mL乙腈中。随后,分批加入8.56 g(2.0当量)K3PO4至反应混合物中,并在相同温度下持续搅拌1.5小时。反应完成后,对混合物进行减压过滤,并用77 mL二氯甲烷洗涤滤饼。滤液经减压浓缩后,将所得残余物溶解于77 mL二氯甲烷中,并用去离子水洗涤。有机层经减压浓缩,最终得到6.17 g目标产物7-氯-1-环丙基-6-氟-1,4-二氢-4-氧代-1,8-二氮杂萘-3-羧酸乙酯,产率为98.5%。产物结构经1H NMR(CDCl3,ppm)确认:1.20(4H,m,CH2CH2),1.41(3H,t,J=8 Hz,CH2CH3),3.66(1H,m,NCH),4.41(2H,q,J=8 Hz,CH2CH3),8.44(1H,d,J=4 Hz,C5-H),8.66(1H,s,C2-H)。
参考文献:
[1] Patent: WO2004/56781, 2004, A1. Location in patent: Page 5-6
[2] Tetrahedron Letters, 1988, vol. 29, # 16, p. 1931 - 1934
[3] Patent: WO2004/56781, 2004, A1. Location in patent: Page 15
[4] Journal of Medicinal Chemistry, 1989, vol. 32, # 3, p. 537 - 542
[5] Journal of Medicinal Chemistry, 1992, vol. 35, # 3, p. 518 - 525
